Constitutive androstane receptor mediates the induction of drug metabolism in mouse models of type 1 diabetes
- PMID: 19489075
- PMCID: PMC2721020
- DOI: 10.1002/hep.23025
Constitutive androstane receptor mediates the induction of drug metabolism in mouse models of type 1 diabetes
Abstract
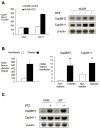
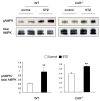
Untreated type 1 diabetes increases hepatic drug metabolism in both human patients and rodent models. We used knockout mice to test the role of the nuclear xenobiotic receptors constitutive androstane receptor (CAR) and pregnane and xenobiotic receptor (PXR) in this process. Streptozotocin-induced diabetes resulted in increased expression of drug metabolizing cytochrome P450s and also increased the clearance of the cytochrome P450 substrate zoxazolamine. This induction was completely absent in Car(-/-) mice, but was not affected by the loss of PXR. Among the many effects of diabetes on the liver, we identified bile acid elevation and activated adenosine monophosphate-activated protein kinase as potential CAR-activating stimuli. Expression of the CAR coactivator peroxisome proliferator-activated receptor gamma coactivator (PGC)-1alpha was also increased in mouse models of type 1 diabetes.
Conclusion: The CAR-dependent induction of drug metabolism in newly diagnosed or poorly managed type 1 diabetes has the potential for significant impact on the efficacy or toxicity of therapeutic agents.
Figures


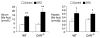
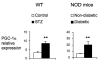
References
-
- Remmer H, Merker HJ. Drug-Induced Changes In The Liver Endoplasmic Reticulum: Association With Drug-Metabolizing Enzymes. Science. 1963;142:1657–1658. - PubMed
-
- Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. - PubMed
-
- Sonoda J, Rosenfeld JM, Xu L, Evans RM, Xie W. A nuclear receptor-mediated xenobiotic response and its implication in drug metabolism and host protection. Curr Drug Metab. 2003;4:59–72. - PubMed
-
- Honkakoski P, Sueyoshi T, Negishi M. Drug-activated nuclear receptors CAR and PXR. Ann Med. 2003;35:172–182. - PubMed
-
- Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab. 2005;6:329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
