Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma
- PMID: 19489099
- PMCID: PMC2771102
- DOI: 10.1002/stem.50
Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma
Abstract
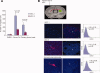
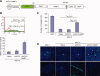
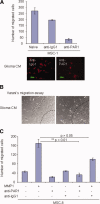
Human mesenchymal stem cells (MSCs) have increasingly been used as cellular vectors for the delivery of therapeutic genes to tumors. However, the precise mechanism of mobilization remains poorly defined. In this study, MSCs that expressed similar cell surface markers and exhibited multilineage differentiation potentials were isolated from various donors. Interestingly, different MSC isolates displayed differential migration ability toward human glioma cells. We hypothesized that distinct molecular signals may be involved in the varied tumor tropisms exhibited by different MSC isolates. To test this hypothesis, gene expression profiles of tumor-trophic MSCs were compared with those of non-tumor-trophic MSCs. Among the various differentially regulated genes, matrix metalloproteinase one (MMP1) gene expression and its protein activities were enhanced by 27-fold and 21-fold, respectively, in highly migrating MSCs compared with poorly migrating MSCs. By contrast, there was no change in the transcriptional levels of other MMPs. Functional inactivation of MMP1 abrogated the migratory potential of MSCs toward glioma-conditioned medium. Conversely, the nonmigratory phenotype of poorly migrating MSC could be rescued in the presence of either recombinant MMP1 or conditioned medium from the highly migrating MSCs. Ectopic expression of MMP1 in these poorly migrating cells also rendered the cells responsive to the signaling cues from the glioma cells in vivo. However, blocking the interaction of MMP1 and its cognate receptor PAR1 effectively diminished the migratory ability of MSCs. Taken together, this study provides, for the first time, supporting evidence that MMP1 is critically involved in the migration capacity of MSCs, acting through the MMP1/PAR1 axis.
Figures


References
-
- Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. - PubMed
-
- Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. - PubMed
-
- Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

