Design, synthesis, and investigation of protein kinase C inhibitors: total syntheses of (+)-calphostin D, (+)-phleichrome, cercosporin, and new photoactive perylenequinones
- PMID: 19489582
- PMCID: PMC2903893
- DOI: 10.1021/ja902324j
Design, synthesis, and investigation of protein kinase C inhibitors: total syntheses of (+)-calphostin D, (+)-phleichrome, cercosporin, and new photoactive perylenequinones
Abstract
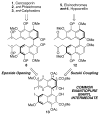
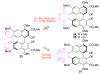
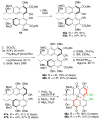
The total syntheses of the PKC inhibitors (+)-calphostin D, (+)-phleichrome, cercosporin, and 10 novel perylenequinones are detailed. The highly convergent and flexible strategy developed employed an enantioselective oxidative biaryl coupling and a double cuprate epoxide opening, allowing the selective syntheses of all the possible stereoisomers in pure form. In addition, this strategy permitted rapid access to a broad range of analogues, including those not accessible from the natural products. These compounds provided a powerful means for evaluation of the perylenequinone structural features necessary to PKC activity. Simpler analogues were discovered with superior PKC inhibitory properties and superior photopotentiation in cancer cell lines relative to the more complex natural products.
Figures








Similar articles
-
Perylenequinone natural products: total syntheses of the diastereomers (+)-phleichrome and (+)-calphostin D by assembly of centrochiral and axial chiral fragments.J Org Chem. 2010 Jan 1;75(1):30-43. doi: 10.1021/jo901384h. J Org Chem. 2010. PMID: 19894745 Free PMC article.
-
Perylenequinone natural products: enantioselective synthesis of the oxidized pentacyclic core.J Org Chem. 2010 Jan 1;75(1):16-29. doi: 10.1021/jo9013832. J Org Chem. 2010. PMID: 19894746 Free PMC article.
-
General approach for the synthesis of chiral perylenequinones via catalytic enantioselective oxidative biaryl coupling.J Am Chem Soc. 2003 Jun 11;125(23):6856-7. doi: 10.1021/ja027745k. J Am Chem Soc. 2003. PMID: 12783524
-
Biosynthesis of Natural and Unnatural Perylenequinones for Drug Development.ChemMedChem. 2024 Oct 16;19(20):e202400295. doi: 10.1002/cmdc.202400295. Epub 2024 Aug 20. ChemMedChem. 2024. PMID: 38943237 Review.
-
Naturally Occurring Partially Reduced Perylenequinones from Fungi.J Nat Prod. 2022 Sep 23;85(9):2236-2250. doi: 10.1021/acs.jnatprod.2c00368. Epub 2022 Sep 13. J Nat Prod. 2022. PMID: 36098709 Review.
Cited by
-
Perylenequinone natural products: total syntheses of the diastereomers (+)-phleichrome and (+)-calphostin D by assembly of centrochiral and axial chiral fragments.J Org Chem. 2010 Jan 1;75(1):30-43. doi: 10.1021/jo901384h. J Org Chem. 2010. PMID: 19894745 Free PMC article.
-
Molecular Characterization of the Cercosporin Biosynthetic Pathway in the Fungal Plant Pathogen Cercospora nicotianae.J Am Chem Soc. 2016 Mar 30;138(12):4219-28. doi: 10.1021/jacs.6b00633. Epub 2016 Mar 16. J Am Chem Soc. 2016. PMID: 26938470 Free PMC article.
-
A Novel Derivative of (-)mycousnine Produced by the Endophytic Fungus Mycosphaerella nawae, Exhibits High and Selective Immunosuppressive Activity on T Cells.Front Microbiol. 2017 Jul 5;8:1251. doi: 10.3389/fmicb.2017.01251. eCollection 2017. Front Microbiol. 2017. PMID: 28725220 Free PMC article.
-
Structural reassignment of a marine metabolite from a binaphthalenetetrol to a tetrabrominated diphenyl ether.J Nat Prod. 2012 Jun 22;75(6):1125-9. doi: 10.1021/np300141t. Epub 2012 Jun 12. J Nat Prod. 2012. PMID: 22690692 Free PMC article.
-
Asymmetric Synthesis of Gonytolide A: Strategic Use of an Aryl Halide Blocking Group for Oxidative Coupling.J Am Chem Soc. 2018 May 9;140(18):5969-5975. doi: 10.1021/jacs.8b02535. Epub 2018 Apr 25. J Am Chem Soc. 2018. PMID: 29658717 Free PMC article.
References
-
- Li X, Yang J, Kozlowski MC. Org Lett. 2001;3:1137–1140. - PubMed
- Kozlowski MC, Li X, Carroll PJ, Xu Z. Organometallics. 2002;21:4513–4522.
- Xie X, Phuan PW, Kozlowski MC. Angew Chem, Int Ed. 2003;42:2168–2170. - PubMed
- Li X, Hewgley JB, Mulrooney C, Yang J, Kozlowski MC. J Org Chem. 2003;68:5500–5511. - PubMed
- Morgan BJ, Xie X, Phuan PW, Kozlowski MC. J Org Chem. 2007;72:6171–6182. - PubMed
- Hewgley JB, Stahl SS, Kozlowski MC. J Am Chem Soc. 2008;130:12232–12233. - PMC - PubMed
-
- Mulrooney CA, Li X, DiVirgilio ES, Kozlowski MC. J Am Chem Soc. 2003;125:6856–6857. - PubMed
-
- O’Brien EM, Morgan BJ, Kozlowski MC. Angew Chem Int Ed. 2008;47:6877–6880. - PubMed
-
- Broka CA. Tetrahedron Lett. 1991;32:859–862.
- Hauser FM, Sengupta D, Corlett SA. J Org Chem. 1994;59:1967–1969.
- Coleman RS, Grant EB. J Am Chem Soc. 1994;116:8795–8796.
- Coleman RS, Grant EB. J Am Chem Soc. 1995;117:10889–10904.
- Merlic CA, Aldrich CC, Albaneze-Walker J, Saghatelian A. J Am Chem Soc. 2000;122:3224–3225. - PMC - PubMed
- Merlic CA, Aldrich CC, Albaneze-Walker J, Saghatelian A, Mammen J. J Org Chem. 2001;66:1297–1309. - PubMed
-
- Nasini G, Merlini L, Andreetti GD, Bocelli G, Sgarabotto P. Tetrahedron. 1982;38:2787–2796.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

