Biosynthesis of phosphonic and phosphinic acid natural products
- PMID: 19489722
- PMCID: PMC2729427
- DOI: 10.1146/annurev.biochem.78.091707.100215
Biosynthesis of phosphonic and phosphinic acid natural products
Abstract
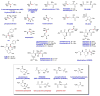
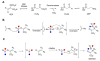
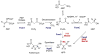
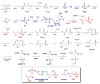
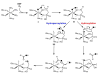
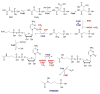
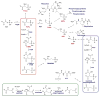
Natural products containing carbon-phosphorus bonds (phosphonic and phosphinic acids) have found widespread use in medicine and agriculture. Recent years have seen a renewed interest in the biochemistry and biology of these compounds with the cloning of the biosynthetic gene clusters for several family members. This review discusses the commonalities and differences in the molecular logic that lie behind the biosynthesis of these compounds. The current knowledge regarding the metabolic pathways and enzymes involved in the production of a number of natural products, including the approved antibiotic fosfomycin, the widely used herbicide phosphinothricin (PT), and the clinical candidate for treatment of malaria FR-900098, is presented. Many of the enzymes involved in the biosynthesis of these compounds catalyze chemically and biologically unprecedented transformations, and a wealth of new biochemistry has been revealed through their study. These investigations have also suggested new strategies for natural product discovery.
Figures


References
-
- Horiguchi M. Occurence, identification and properties of phosphonic and phosphinic acids. In: Hori T, Horiguchi T, Hayashi A, editors. Biochemistry of natural C-P compounds. Chapter 3. Shiga, Japan: Japanese Association for Research on the Biochemistry of C-P Compounds; 1984. pp. 24–52.
-
- Kafarski P, Lejczak B. Aminophosphonic acids of potential medical importance. Curr Med Chem Anti-Cancer Agents. 2001;1:301–12. - PubMed
-
- Horiguchi M, Kandatsu M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature. 1959;184 12:901–2. The first observation of a C-P compound in a living system. - PubMed
-
- Miceli MV, Henderson TO, Myers TC. 2-Aminoethylphosphonic Acid Metabolism During Embryonic Development of the Planorbid Snail Helisoma. Science. 1980;209:1245–47. - PubMed
-
- Quin LD. The presences of compounds with a carbon-phosphorus bond in some marine invertebrates. Biochemistry. 1965;4:324–30.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

