Degradation of activated protein kinases by ubiquitination
- PMID: 19489726
- PMCID: PMC2776765
- DOI: 10.1146/annurev.biochem.013008.092711
Degradation of activated protein kinases by ubiquitination
Abstract
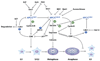
Protein kinases are important regulators of intracellular signal transduction pathways and play critical roles in diverse cellular functions. Once a protein kinase is activated, its activity is subsequently downregulated through a variety of mechanisms. Accumulating evidence indicates that the activation of protein kinases commonly initiates their downregulation via the ubiquitin/proteasome pathway. Failure to regulate protein kinase activity or expression levels can cause human diseases.
Figures




References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. - PubMed
-
- Hunter T. Signaling–2000 and beyond. Cell. 2000;100:113–127. - PubMed
-
- Oda A, Druker BJ, Ariyoshi H, Smith M, Salzman EW. pp60src is an endogenous substrate for calpain in human blood platelets. J. Biol. Chem. 1993;268:12603–12608. - PubMed
-
- Karni R, Levitzki A. pp60(cSrc) is a caspase-3 substrate and is essential for the transformed phenotype of A431 cells. Mol. Cell Biol. Res. Commun. 2000;3:98–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

