Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology
- PMID: 19490023
- PMCID: PMC2914561
- DOI: 10.1111/j.1460-9568.2009.06786.x
Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology
Abstract
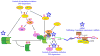
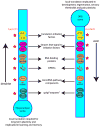
The increased sensitivity of peripheral pain-sensing neurons, or nociceptors, is a major cause of the sensation of pain that follows injury. This plasticity is thought to contribute to the maintenance of chronic pain states. Although we have a broad knowledge of the factors that stimulate changes in nociceptor sensitivity, the cellular mechanisms that underlie this plasticity are still poorly understood; however, they are likely to involve changes in gene expression required for the phenotypic and functional changes seen in nociceptive neurons after injury. While the regulation of gene expression at the transcriptional level has been studied extensively, the regulation of protein synthesis, which is also a tightly controlled process, has only recently received more attention. Despite the established role of protein synthesis in the plasticity of neuronal cell bodies and dendrites, little attention has been paid to the role of translation control in mature undamaged axons. In this regard, several recent studies have demonstrated that the control of protein synthesis within the axonal compartment is crucial for the normal function and regulation of sensitivity of nociceptors. Pathways and proteins regulating this process, such as the mammalian target of rapamycin signaling cascade and the fragile X mental retardation protein, have recently been identified. We review here recent evidence for the regulation of protein synthesis within a nociceptor's axonal compartment and its contribution to this neuron's plasticity. We believe that an increased understanding of this process will lead to the identification of novel targets for the treatment of chronic pain.
Figures
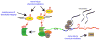
Similar articles
-
Axonal protein synthesis: a potential target for pain relief?Curr Opin Pharmacol. 2012 Feb;12(1):42-8. doi: 10.1016/j.coph.2011.10.005. Epub 2011 Oct 25. Curr Opin Pharmacol. 2012. PMID: 22033338 Review.
-
The brinker repressor system regulates injury-induced nociceptive sensitization in Drosophila melanogaster.Mol Pain. 2021 Jan-Dec;17:17448069211037401. doi: 10.1177/17448069211037401. Mol Pain. 2021. PMID: 34399634 Free PMC article.
-
The MNK-eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain.J Neurosci. 2017 Aug 2;37(31):7481-7499. doi: 10.1523/JNEUROSCI.0220-17.2017. Epub 2017 Jul 3. J Neurosci. 2017. PMID: 28674170 Free PMC article.
-
Characterization of Fragile X Mental Retardation Protein expression in human nociceptors and their axonal projections to the spinal dorsal horn.J Comp Neurol. 2023 May;531(7):814-835. doi: 10.1002/cne.25463. Epub 2023 Feb 20. J Comp Neurol. 2023. PMID: 36808110 Free PMC article.
-
Translational Control Mechanisms in Persistent Pain.Trends Neurosci. 2018 Feb;41(2):100-114. doi: 10.1016/j.tins.2017.11.006. Trends Neurosci. 2018. PMID: 29249459 Free PMC article. Review.
Cited by
-
Pharmacological Manipulation of Translation as a Therapeutic Target for Chronic Pain.Pharmacol Rev. 2021 Jan;73(1):59-88. doi: 10.1124/pharmrev.120.000030. Pharmacol Rev. 2021. PMID: 33203717 Free PMC article. Review.
-
Deciphering the molecular landscape of human peripheral nerves: implications for diabetic peripheral neuropathy.bioRxiv [Preprint]. 2024 Jun 16:2024.06.15.599167. doi: 10.1101/2024.06.15.599167. bioRxiv. 2024. PMID: 38915676 Free PMC article. Preprint.
-
Inhibition of Poly(A)-binding protein with a synthetic RNA mimic reduces pain sensitization in mice.Nat Commun. 2018 Jan 2;9(1):10. doi: 10.1038/s41467-017-02449-5. Nat Commun. 2018. PMID: 29295980 Free PMC article.
-
Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity.Mol Pain. 2014 Jul 4;10:45. doi: 10.1186/1744-8069-10-45. Mol Pain. 2014. PMID: 24993495 Free PMC article.
-
Cdk5: An Emerging Kinase in Pain Signaling.Brain Disord Ther. 2013 Jul;2013(Suppl 1):003. doi: 10.4172/2168-975X.S1-003. Epub 2012 Oct 3. Brain Disord Ther. 2013. PMID: 28316897 Free PMC article.
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Alvarez J. The autonomous axon: a model based on local synthesis of proteins. Biol Res. 2001;34:103–109. - PubMed
-
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

