Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice
- PMID: 19490051
- PMCID: PMC3724531
- DOI: 10.1111/j.1528-1167.2009.02163.x
Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice
Abstract
Introduction: Seizures are known to perturb circadian rhythms in humans as well as in animal models of epilepsy. However, it is unknown whether treatment of the underlying epilepsy restores normal biologic rhythms. We asked whether: (1) seizure activity is characterized by diurnal rhythmicity, (2) chronically epileptic mice exhibit impaired rest-activity rhythms, and (3) treatment with the anticonvulsant ketogenic diet (KD) improves such perturbations.
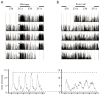
Methods: Chronically epileptic Kcna1-null mice were fed either a standard diet (SD) or KD for 4 weeks and subjected to continuous video-EEG (electroencephalography) and actigraphy monitoring for 3-5 days to assess seizure activity and rest-activity cycles.
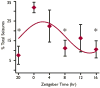
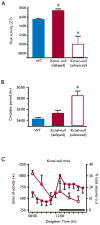
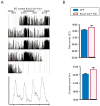
Results: Seizure activity in Kcna1-null mice demonstrated diurnal rhythmicity, peaking at zeitgeber (ZT)2.30 +/- 1.52. Rest-activity rhythms of epileptic mice were significantly disrupted. Whereas locomotor activity for wild-type mice peaked at ZT15.45 +/- 0.28 (ZT14:26-ZT16:51), peak activity of epileptic mice was more unpredictable, occurring over a 12.4 h range (ZT06:33-ZT18:57). In six of nine epileptic mice, peak activity was delayed to ZT17.42 +/- 0.38, whereas peak activity was advanced to ZT10.00 +/- 1.26 in the remaining mice. Treatment with the KD abolished seizure periodicity and restored the rest-activity rhythm to values resembling those of wild-type mice (i.e., activity peaking at ZT16.73 +/- 0.67).
Conclusions: Kcna1-null mice experience seizures with 24-h periodicity and impaired circadian behavior. KD reduces the number and periodicity of seizures and restores normal behavioral rhythms, suggesting that this nonpharmacologic therapy may benefit biologic rhythm disturbances in epileptic patients.
Conflict of interest statement
Figures
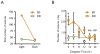
References
-
- Bastlund JF, Jennum P, Mohapel P, Penschuck S, Watson WP. Spontaneous epileptic rats show changes in sleep architecture and hypothalamic pathology. Epilepsia. 2005;46:934–8. - PubMed
-
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–62. - PubMed
-
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

