Relative seroprevalence of human herpes viruses in patients with chronic lymphocytic leukaemia
- PMID: 19490058
- PMCID: PMC3709071
- DOI: 10.1111/j.1365-2362.2009.02131.x
Relative seroprevalence of human herpes viruses in patients with chronic lymphocytic leukaemia
Abstract
Background: Herpes virus infections may have a significant role in chronic lymphocytic leukaemia (CLL) due to their ability to modulate the host's immune system.
Materials and methods: We examined the seroprevalence of four herpes viruses [Cytomegalovirus (CMV), Epstein-Barr Virus (EBV), human herpes virus (HHV)-6 and -7] in a cohort of European CLL patients (cohort 1, n = 100) in relation to the immunoglobulin variable heavy (IGHV) chain gene use and compared serological results with those obtained from age- and gender-matched healthy adults (n = 100).
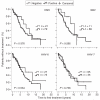
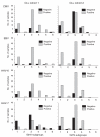
Results: CMV-seroprevalence was significantly higher in CLL cohort 1 (79%) than in the control cohort (57%, P = 0.001); the seroprevalence of EBV (89% vs. 94%), HHV-6 (73% vs. 60%), or HHV-7 (35% vs. 35%) was not. In CLL cohort 1, use of IGHV3-30 was more prevalent among CMV-seropositive and of IGHV3-21 among HHV-7-seronegative cases. To investigate the generalizability of these findings, we investigated the herpes virus seroprevalence in a second cohort of age-matched CLL patients from a different geographical area (USA, n = 100, cohort 2). In cohort 2, CMV-seroprevalence was comparable with that of the control cohort (53%). Seroprevalence of EBV, HHV-6 and HHV-7 were 85%, 88% and 73% respectively. In CLL cohort 2, use of IGHV3-30 or IGHV3-21 was not associated with any of the herpes viruses investigated.
Conclusions: CMV-seropositivity is associated with CLL in selected patient cohorts. However, the considerable variation in herpes virus-specific seropositivity between geographically distinct CLL cohorts indicates that seropositivity for any of the four human herpes viruses investigated is not generally associated with CLL.
Figures
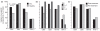

Similar articles
-
Seroprevalence of HHV-8, CMV, and EBV among the general population in Ghana, West Africa.BMC Infect Dis. 2008 Aug 18;8:111. doi: 10.1186/1471-2334-8-111. BMC Infect Dis. 2008. PMID: 18706107 Free PMC article.
-
[High seroprevalence of cytomegalovirus, herpes simplex type 1 virus and Epstein Barr virus infection among human immunodeficiency virus-infected adults].Rev Med Chil. 2010 Jul;138(7):809-14. doi: 10.4067/s0034-98872010000700003. Rev Med Chil. 2010. PMID: 21043074 Spanish.
-
Seroepidemiology of Epstein-Barr virus and cytomegalovirus among Israeli male young adults.Ann Epidemiol. 2012 Nov;22(11):783-8. doi: 10.1016/j.annepidem.2012.06.099. Epub 2012 Jul 24. Ann Epidemiol. 2012. PMID: 22831994
-
Herpes Group Viruses: a Seroprevalence Study in Hemodialysis Patients.Acta Clin Croat. 2017 Jun;56(2):255-261. doi: 10.20471/acc.2017.56.02.08. Acta Clin Croat. 2017. PMID: 29485792
-
B-cell receptor, clinical course and prognosis in chronic lymphocytic leukaemia: the growing saga of the IGHV3 subgroup gene usage.Br J Haematol. 2011 Apr;153(1):3-14. doi: 10.1111/j.1365-2141.2010.08440.x. Epub 2011 Feb 8. Br J Haematol. 2011. PMID: 21303354 Review.
Cited by
-
β-HHVs and HHV-8 in Lymphoproliferative Disorders.Mediterr J Hematol Infect Dis. 2011;3(1):e2011043. doi: 10.4084/MJHID.2011.043. Epub 2011 Oct 24. Mediterr J Hematol Infect Dis. 2011. PMID: 22110893 Free PMC article.
-
EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells--a new option for the treatment of B-CLL.PLoS One. 2011;6(10):e25294. doi: 10.1371/journal.pone.0025294. Epub 2011 Oct 10. PLoS One. 2011. PMID: 22022385 Free PMC article.
-
Study of the Associations Between TT Virus Single and Mixed Infections With Leukemia.Jundishapur J Microbiol. 2015 Apr 18;8(4):e18212. doi: 10.5812/jjm.8(4)2015.18212. eCollection 2015 Apr. Jundishapur J Microbiol. 2015. PMID: 26034552 Free PMC article.
-
Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.Front Immunol. 2019 Aug 14;10:1946. doi: 10.3389/fimmu.2019.01946. eCollection 2019. Front Immunol. 2019. PMID: 31475007 Free PMC article.
-
Pathogen-specific B-cell receptors drive chronic lymphocytic leukemia by light-chain-dependent cross-reaction with autoantigens.EMBO Mol Med. 2017 Nov;9(11):1482-1490. doi: 10.15252/emmm.201707732. EMBO Mol Med. 2017. PMID: 28899929 Free PMC article.
References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. - PubMed
-
- Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78:374–7. - PubMed
-
- Widhopf GF, Brinson DC, Kipps TJ, Tighe H. Transgenic expression of a human polyreactive Ig expressed in chronic lymphocytic leukemia generates memory-type B cells that respond to nonspecific immune activation. J Immunol. 2004;172:2092–9. - PubMed

