WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease
- PMID: 19490092
- PMCID: PMC2755595
- DOI: 10.1111/j.1460-9568.2009.06764.x
WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease
Abstract
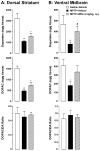
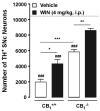
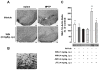
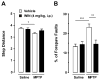
Parkinson's disease (PD) is characterized by the progressive loss of nigrostriatal dopamine neurons leading to motor disturbances and cognitive impairment. Current pharmacotherapies relieve PD symptoms temporarily but fail to prevent or slow down the disease progression. In this study, we investigated the molecular mechanisms by which the non-selective cannabinoid receptor agonist WIN55,212-2 (WIN) protects mouse nigrostriatal neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity and neuroinflammation. Stereological analyses showed that chronic treatment with WIN (4 mg/kg, intraperitoneal), initiated 24 h after MPTP administration, protected against MPTP-induced loss of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta independently of CB1 cannabinoid receptor activation. The neuroprotective effect of WIN was accompanied by increased dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra pars compacta and dorsal striatum of MPTP-treated mice. At 3 days post-MPTP, we found significant microglial activation and up-regulation of CB2 cannabinoid receptors in the ventral midbrain. Treatment with WIN or the CB2 receptor agonist JWH015 (4 mg/kg, intraperitoneal) reduced MPTP-induced microglial activation, whereas genetic ablation of CB2 receptors exacerbated MPTP systemic toxicity. Furthermore, chronic WIN reversed MPTP-associated motor deficits, as revealed by the analysis of forepaw step width and percentage of faults using the inverted grid test. In conclusion, our data indicate that agonism at CB2 cannabinoid receptors protects against MPTP-induced nigrostriatal degeneration by inhibiting microglial activation/infiltration and suggest that CB2 receptors represent a new therapeutic target to slow the degenerative process occurring in PD.
Figures


Similar articles
-
Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation.J Immunol. 2011 Dec 15;187(12):6508-17. doi: 10.4049/jimmunol.1102435. Epub 2011 Nov 11. J Immunol. 2011. PMID: 22079984
-
Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease.J Neuroinflammation. 2010 Nov 23;7:83. doi: 10.1186/1742-2094-7-83. J Neuroinflammation. 2010. PMID: 21092260 Free PMC article.
-
EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: role of oxidative stress.Eur J Neurosci. 2008 Jul;28(1):41-50. doi: 10.1111/j.1460-9568.2008.06314.x. Eur J Neurosci. 2008. PMID: 18662333
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
-
Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol.Parkinsonism Relat Disord. 2008;14 Suppl 2(Suppl 2):S112-5. doi: 10.1016/j.parkreldis.2008.04.012. Epub 2008 Jun 27. Parkinsonism Relat Disord. 2008. PMID: 18585085 Free PMC article. Review.
Cited by
-
Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson's Disease.Front Neurosci. 2016 Aug 2;10:321. doi: 10.3389/fnins.2016.00321. eCollection 2016. Front Neurosci. 2016. PMID: 27531971 Free PMC article.
-
Modulation of cannabinoid receptor 2 alters neuroinflammation and reduces formation of alpha-synuclein aggregates in a rat model of nigral synucleinopathy.J Neuroinflammation. 2024 Sep 27;21(1):240. doi: 10.1186/s12974-024-03221-5. J Neuroinflammation. 2024. PMID: 39334169 Free PMC article.
-
The impact of cannabinoid type 2 receptors (CB2Rs) in neuroprotection against neurological disorders.Acta Pharmacol Sin. 2020 Dec;41(12):1507-1518. doi: 10.1038/s41401-020-00530-2. Epub 2020 Oct 6. Acta Pharmacol Sin. 2020. PMID: 33024239 Free PMC article. Review.
-
The Neuroprotective Effects of the CB2 Agonist GW842166x in the 6-OHDA Mouse Model of Parkinson's Disease.Cells. 2021 Dec 16;10(12):3548. doi: 10.3390/cells10123548. Cells. 2021. PMID: 34944056 Free PMC article.
-
Quality of Life and a Surveillant Endocannabinoid System.Front Neurosci. 2021 Oct 28;15:747229. doi: 10.3389/fnins.2021.747229. eCollection 2021. Front Neurosci. 2021. PMID: 34776851 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

