Use of nicotine substitute prescribed at hourly plus ab libitum intake or ad libitum for heavy smokers willing to quit: a randomized controlled trial
- PMID: 19490626
- PMCID: PMC2698911
- DOI: 10.1186/1747-597X-4-12
Use of nicotine substitute prescribed at hourly plus ab libitum intake or ad libitum for heavy smokers willing to quit: a randomized controlled trial
Abstract
Objective: To assess the impact of instructional guidance in the regular use of use nicotine nasal spray (NNS) on the true use of NNS during the first three weeks of smoking cessation for heavy smokers who are willing to quit.
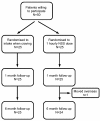
Methods: This randomized, open, controlled trial included 50 patients who were heavy smokers, were willing to quit, and attending an academic outpatient clinic in Western Switzerland. Patients were randomised to instruction on NNS use as "ad libitum" (administration whenever cravings appear; control group) or to use NNS when craving appears and at least every hour when awake (intervention group). Intakes were monitored using an electronic device fixed in the spray unit (MDILog) during the first three weeks of use. Self reported abstinence from smoking at six months was confirmed by expired-air carbon monoxide. Using intention-to-treat analysis, random-effect GLS regression was used to calculate the mean difference of daily doses between groups controlling for lack of independence between measures from the same individual.
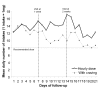
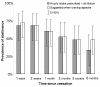
Results: One patient was lost to follow-up. At baseline randomization, the group receiving instruction to use NNS hourly included more women, patients with previous desires to quit, and patients with more psychiatric comorbidities and less somatic complaints compared to the group instructed to use NNS with cravings (group imbalance). Both groups self-administered more than the daily recommended dosage of 8 uses. Mean daily usage was 13.6 dose/day and 11.1 dose/day for the group instructed to use NNS hourly and with cravings, respectively. Adjusting for baseline imbalance, the increased daily doses in the intervention group (hourly use) remained nonsignificant compared to ad libitum use (-0.5 dose/day; CI 95% -6.2; 5.3, from day 1 to day 7; and 2.3 dose/day; CI 95% -5.4; 10.0, from day 8 to day 21). Instructing patients to use the NNS daily had no effect on smoking cessation at six months (RR = 0.69; CI 95% 0.34; 1.39).
Conclusion: Heavy smokers willing to quit use NNS frequently, regardless of the instructions given. Recommending the use of NNS only when craving appears for heavy smokers willing to quit seems acceptable compared to prescribing hourly administration.
Trial registration: ClinicalTrials.gov: NCT00861276.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

