The tortoise and the hair: slow-cycling cells in the stem cell race
- PMID: 19490891
- PMCID: PMC2716122
- DOI: 10.1016/j.cell.2009.05.002
The tortoise and the hair: slow-cycling cells in the stem cell race
Abstract
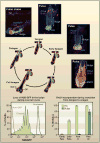
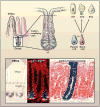
Given the importance of stem cells to adult tissues, it has long been postulated that stem cells divide infrequently to preserve their long-term proliferation potential and to prevent the acquisition of errors during DNA replication. Yet, some stem cells must be able to continually churn out progeny in tissues that rapidly turn over or are subject to sudden injuries or growth spurts. This Review explores the challenges that mammalian stem cells face in balancing the competing demands of proliferation and differentiation in tissues.
Figures


References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. IV. Effects of resecting 30% of the small intestine. Am J Anat. 1981;160:93–103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

