The control of food intake: behavioral versus molecular perspectives
- PMID: 19490904
- PMCID: PMC3090647
- DOI: 10.1016/j.cmet.2009.04.007
The control of food intake: behavioral versus molecular perspectives
Abstract
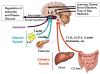
To meet the continuous demand for energy, organisms use diverse signals to match food intake with energy needs. This paper reviews the effect of satiation signals and adiposity signals on food intake, including how they interact in the brain and how their influence changes with experience. Whereas meal initiation is influenced by external environmental factors, meal size is influenced by an array of signals that can be partitioned according to their reliability in indicating caloric content of food. It is argued that the malleability of satiation signals renders them poor candidates as pharmacological targets to control body weight.
Figures


References
-
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. - PubMed
-
- Adolph EF. Urges to eat and drink in rats. Am J Physiol. 1947;151:110–125. - PubMed
-
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. - PubMed
-
- Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89:472–476. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

