GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation
- PMID: 19491097
- PMCID: PMC2740420
- DOI: 10.1074/jbc.M109.015164
GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation
Abstract
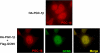
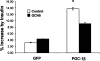
Changes in expression levels of genes encoding for proteins that control metabolic pathways are essential to maintain nutrient and energy homeostasis in individual cells as well as in organisms. An important regulated step in this process is accomplished through covalent chemical modifications of proteins that form complexes with the chromatin of gene promoters. The peroxisome proliferators gamma co-activator 1 (PGC-1) family of transcriptional co-activators comprises important components of a number of these complexes and participates in a large array of glucose and lipid metabolic adaptations. Here, we show that PGC-1beta is acetylated on at least 10 lysine residues distributed along the length of the protein by the acetyl transferase general control of amino-acid synthesis (GCN5) and that this acetylation reaction is reversed by the deacetylase sirtuin 1 (SIRT1). GCN5 strongly interacts with PGC-1beta and represses its transcriptional activity associated with transcription factors such as ERRalpha, NRF-1, and HNF4alpha, however acetylation and transcriptional repression do not occur when a catalytically inactive GCN5 is co-expressed. Transcriptional repression coincides with PGC-1beta redistribution to nuclear foci where it co-localizes with GCN5. Furthermore, knockdown of GCN5 ablates PGC-1beta acetylation and increases transcriptional activity. In primary skeletal muscle cells, PGC-1beta induction of endogenous target genes, including MCAD and GLUT4, is largely repressed by GCN5. Functionally, this translates to a blunted response to PGC-1beta-induced insulin-mediated glucose transport. These results suggest that PGC-1beta acetylation by GCN5 might be an important step in the control of glucose and lipid pathways and its dysregulation could contribute to metabolic diseases.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

