Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane
- PMID: 19491101
- PMCID: PMC2740440
- DOI: 10.1074/jbc.M109.021881
Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane
Abstract
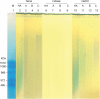
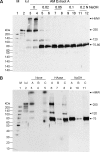
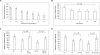
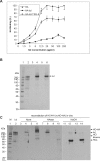
Clinically, amniotic membrane (AM) suppresses inflammation, scarring, and angiogenesis. AM contains abundant hyaluronan (HA) but its function in exerting these therapeutic actions remains unclear. Herein, AM was extracted sequentially with buffers A, B, and C, or separately by phosphate-buffered saline (PBS) alone. Agarose gel electrophoresis showed that high molecular weight (HMW) HA (an average of approximately 3000 kDa) was predominantly extracted in isotonic Extract A (70.1 +/- 6.0%) and PBS (37.7 +/- 3.2%). Western blot analysis of these extracts with hyaluronidase digestion or NaOH treatment revealed that HMW HA was covalently linked with the heavy chains (HCs) of inter-alpha-inhibitor (IalphaI) via a NaOH-sensitive bond, likely transferred by the tumor necrosis factor-alpha stimulated gene-6 protein (TSG-6). This HC.HA complex (nHC*HA) could be purified from Extract PBS by two rounds of CsCl/guanidine HCl ultracentrifugation as well as in vitro reconstituted (rcHC*HA) by mixing HMW HA, serum IalphaI, and recombinant TSG-6. Consistent with previous reports, Extract PBS suppressed transforming growth factor-beta1 promoter activation in corneal fibroblasts and induced mac ro phage apoptosis. However, these effects were abolished by hyaluronidase digestion or heat treatment. More importantly, the effects were retained in the nHC*HA or rcHC*HA. These data collectively suggest that the HC*HA complex is the active component in AM responsible in part for clinically observed anti-inflammatory and anti-scarring actions.
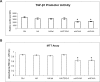
Figures


References
-
- Tammi M. I., Day A. J., Turley E. A. (2002) J. Biol. Chem. 277, 4581–4584 - PubMed
-
- Hascall V. C., Majors A. K., De La Motte C. A., Evanko S. P., Wang A., Drazba J. A., Strong S. A., Wight T. N. (2004) Biochim. Biophys. Acta 1673, 3–12 - PubMed
-
- Rooney P., Wang M., Kumar P., Kumar S. (1993) J. Cell Sci. 105, 213–218 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

