Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct
- PMID: 19491102
- PMCID: PMC2742857
- DOI: 10.1074/jbc.M109.020073
Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct
Abstract
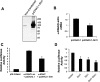
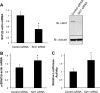
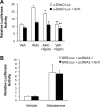
Aldosterone increases renal tubular Na+ absorption in large part by increasing transcription of the epithelial Na(+) channel alpha-subunit (alpha-ENaC) expressed in the apical membrane of collecting duct principal cells. We recently reported that a complex containing the histone H3K79 methyltransferase disruptor of telomeric silencing-1 (Dot1) associates with and represses the alpha-ENaC promoter in mouse inner medullary collecting duct mIMCD3 cells, and that aldosterone acts to disrupt this complex and its inhibitory effects (Zhang, W., Xia, X., Reisenauer, M. R., Rieg, T., Lang, F., Kuhl, D., Vallon, V., and Kone, B. C. (2007) J. Clin. Invest. 117, 773-783). Here we demonstrate that the NAD(+)-dependent deacetylase sirtuin 1 (Sirt1) functionally and physically interacts with Dot1 to enhance the distributive activity of Dot1 on H3K79 methylation and thereby represses alpha-ENaC transcription in mIMCD3 cells. Sirt1 overexpression inhibited basal alpha-ENaC mRNA expression and alpha-ENaC promoter activity, surprisingly in a deacetylase-independent manner. The ability of Sirt1 to inhibit alpha-ENaC transcription was retained in a truncated Sirt1 construct expressing only its N-terminal domain. Conversely, Sirt1 knockdown enhanced alpha-ENaC mRNA levels and alpha-ENaC promoter activity, and inhibited global H3K79 methylation, particularly H3K79 trimethylation, in chromatin associated with the alpha-ENaC promoter. Sirt1 and Dot1 co-immunoprecipitated from mIMCD3 cells and colocalized in the nucleus. Sirt1 immunoprecipitated from chromatin associated with regions of the alpha-ENaC promoter known to associate with Dot1. Aldosterone inhibited Sirt1 association at two of these regions, as well as Sirt1 mRNA expression, in a coordinate manner with induction of alpha-ENaC transcription. Overexpressed Sirt1 inhibited aldosterone induction of alpha-ENaC transcription independent of effects on mineralocorticoid receptor trans-activation. These data identify Sirt1 as a novel modulator of alpha-ENaC, Dot1, and the aldosterone signaling pathway.
Figures


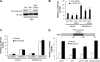

Similar articles
-
Dot1a contains three nuclear localization signals and regulates the epithelial Na+ channel (ENaC) at multiple levels.Am J Physiol Renal Physiol. 2010 Jul;299(1):F63-76. doi: 10.1152/ajprenal.00105.2010. Epub 2010 Apr 28. Am J Physiol Renal Physiol. 2010. PMID: 20427473 Free PMC article.
-
Aldosterone-sensitive repression of ENaCalpha transcription by a histone H3 lysine-79 methyltransferase.Am J Physiol Cell Physiol. 2006 Mar;290(3):C936-46. doi: 10.1152/ajpcell.00431.2005. Epub 2005 Oct 19. Am J Physiol Cell Physiol. 2006. PMID: 16236820 Free PMC article.
-
CREB trans-activation of disruptor of telomeric silencing-1 mediates forskolin inhibition of CTGF transcription in mesangial cells.Am J Physiol Renal Physiol. 2010 Mar;298(3):F617-24. doi: 10.1152/ajprenal.00636.2009. Epub 2010 Jan 6. Am J Physiol Renal Physiol. 2010. PMID: 20053791 Free PMC article.
-
Epigenetics and the control of the collecting duct epithelial sodium channel.Semin Nephrol. 2013 Jul;33(4):383-91. doi: 10.1016/j.semnephrol.2013.05.010. Semin Nephrol. 2013. PMID: 24011580 Free PMC article. Review.
-
Epigenetics and the control of epithelial sodium channel expression in collecting duct.Kidney Int. 2009 Feb;75(3):260-7. doi: 10.1038/ki.2008.475. Epub 2008 Sep 24. Kidney Int. 2009. PMID: 18818687 Free PMC article. Review.
Cited by
-
Physical and functional interaction of Rnf2 with Af9 regulates basal and aldosterone-stimulated transcription of the α-ENaC gene in a renal collecting duct cell line.Biosci Rep. 2013 Oct 25;33(5):e00076. doi: 10.1042/BSR20130086. Biosci Rep. 2013. PMID: 24070375 Free PMC article.
-
Dot1 binding induces chromatin rearrangements by histone methylation-dependent and -independent mechanisms.Epigenetics Chromatin. 2011 Feb 3;4(1):2. doi: 10.1186/1756-8935-4-2. Epigenetics Chromatin. 2011. PMID: 21291527 Free PMC article.
-
Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors.J Am Soc Nephrol. 2020 May;31(5):907-919. doi: 10.1681/ASN.2020010010. Epub 2020 Apr 10. J Am Soc Nephrol. 2020. PMID: 32276962 Free PMC article. Review.
-
Effects of genetic variation in H3K79 methylation regulatory genes on clinical blood pressure and blood pressure response to hydrochlorothiazide.J Transl Med. 2012 Mar 22;10:56. doi: 10.1186/1479-5876-10-56. J Transl Med. 2012. PMID: 22440088 Free PMC article. Clinical Trial.
-
Human genetics of diabetic retinopathy.J Endocrinol Invest. 2014 Dec;37(12):1165-74. doi: 10.1007/s40618-014-0172-8. Epub 2014 Sep 9. J Endocrinol Invest. 2014. PMID: 25201002 Review.
References
-
- Rossier B. C., Pradervand S., Schild L., Hummler E. (2002) Annu. Rev. Physiol. 64, 877–897 - PubMed
-
- Schild L. (1996) Nephrologie 17, 395–400 - PubMed
-
- Mick V. E., Itani O. A., Loftus R. W., Husted R. F., Schmidt T. J., Thomas C. P. (2001) Mol. Endocrinol. 15, 575–588 - PubMed
-
- Husted R. F., Volk K. A., Sigmund R. D., Stokes J. B. (2007) Am. J. Physiol. Renal Physiol. 293, F813–F820 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

