Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells
- PMID: 19491199
- PMCID: PMC2755540
- DOI: 10.1158/1541-7786.MCR-08-0326
Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells
Abstract
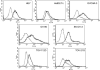
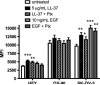
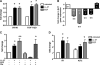
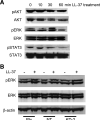
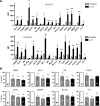
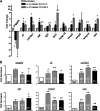
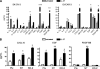
Emerging evidence suggests that the antimicrobial peptide, leucine leucine-37 (LL-37), could play a role in the progression of solid tumors. LL-37 is expressed as the COOH terminus of human cationic antimicrobial protein-18 (hCAP-18) in ovarian, breast, and lung cancers. Previous studies have shown that the addition of LL-37 to various cancer cell lines in vitro stimulates proliferation, migration, and invasion. Similarly, overexpression of hCAP-18/LL-37 in vivo accelerates tumor growth. However, the receptor or receptors through which these processes are mediated have not been thoroughly examined. In the present study, expression of formyl peptide receptor-like 1 (FPRL1) was confirmed on ovarian cancer cells. Proliferation assays indicated that LL-37 does not signal through a G protein-coupled receptor, such as FPRL1, to promote cancer cell growth. By contrast, FPRL1 was required for LL-37-induced invasion through Matrigel. The peptide stimulated mitogen-activated protein kinase and Janus-activated kinase/signal transducers and activators of transcription signaling cascades and led to the significant activation of several transcription factors, through both FPRL1-dependent and FPRL1-independent pathways. Likewise, expression of some LL-37-stimulated genes was attenuated by the inhibition of FPRL1. Increased expression of CXCL10, EGF, and PDGF-BB as well as other soluble factors was confirmed from conditioned medium of LL-37-treated cells. Taken together, these data suggest that LL-37 potentiates a more aggressive behavior from ovarian cancer cells through its interaction with FPRL1.
Figures
Similar articles
-
Human cathelicidin antimicrobial peptide LL-37 promotes lymphangiogenesis in lymphatic endothelial cells through the ERK and Akt signaling pathways.Mol Biol Rep. 2020 Sep;47(9):6841-6854. doi: 10.1007/s11033-020-05741-8. Epub 2020 Sep 4. Mol Biol Rep. 2020. PMID: 32886325
-
FPRL1-mediated induction of superoxide in LL-37-stimulated IMR90 human fibroblast.Arch Biochem Biophys. 2009 Jan 1;481(1):94-100. doi: 10.1016/j.abb.2008.10.026. Epub 2008 Oct 26. Arch Biochem Biophys. 2009. PMID: 18996352
-
The formyl peptide receptor like-1 and scavenger receptor MARCO are involved in glial cell activation in bacterial meningitis.J Neuroinflammation. 2011 Feb 7;8(1):11. doi: 10.1186/1742-2094-8-11. J Neuroinflammation. 2011. PMID: 21299846 Free PMC article.
-
Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in Alzheimer disease.Immunol Res. 2005;31(3):165-76. doi: 10.1385/IR:31:3:165. Immunol Res. 2005. PMID: 15888909 Review.
-
Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30.Curr Top Med Chem. 2009;9(13):1227-36. doi: 10.2174/156802609789753608. Curr Top Med Chem. 2009. PMID: 19807662 Free PMC article. Review.
Cited by
-
Cathelicidin, an antimicrobial peptide produced by macrophages, promotes colon cancer by activating the Wnt/β-catenin pathway.Oncotarget. 2015 Feb 20;6(5):2939-50. doi: 10.18632/oncotarget.2845. Oncotarget. 2015. PMID: 25596747 Free PMC article.
-
Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties.Oncotarget. 2015 Oct 6;6(30):28573-87. doi: 10.18632/oncotarget.5611. Oncotarget. 2015. PMID: 26378047 Free PMC article.
-
Inhibition of formyl peptide receptor 1 activity suppresses tumorigenicity in vivo and attenuates the invasion and migration of lung adenocarcinoma cells under hypoxic conditions in vitro.Ann Transl Med. 2020 Sep;8(18):1174. doi: 10.21037/atm-20-5864. Ann Transl Med. 2020. PMID: 33241023 Free PMC article.
-
Biological Effects of Calceolarioside A as a Natural Compound: Anti-Ovarian Cancer, Anti-Tyrosinase, and Anti-HMG-CoA Reductase Potentials with Molecular Docking and Dynamics Simulation Studies.Mol Biotechnol. 2025 Jan 17. doi: 10.1007/s12033-025-01369-w. Online ahead of print. Mol Biotechnol. 2025. PMID: 39820851
-
Antimicrobial peptide LL-37 promotes the proliferation and invasion of skin squamous cell carcinoma by upregulating DNA-binding protein A.Oncol Lett. 2016 Sep;12(3):1745-1752. doi: 10.3892/ol.2016.4865. Epub 2016 Jul 15. Oncol Lett. 2016. PMID: 27588122 Free PMC article.
References
-
- Coffelt SB, Waterman RS, Florez L, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–9. - PubMed
-
- Heilborn JD, Nilsson MF, Jimenez CI, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–9. - PubMed
-
- von Haussen J, Koczulla R, Shaykhiev R, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

