Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage
- PMID: 19491211
- PMCID: PMC2712783
- DOI: 10.2337/db08-1327
Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage
Abstract
Objective: The aim of the study is to evaluate the effect of nanoparticle-mediated gene delivery of angiogenic inhibitors on retinal inflammation, vascular leakage, and neovascularization in diabetic retinopathy.
Research design and methods: An expression plasmid of plasminogen kringle 5 (K5), a natural angiogenic inhibitor, was encapsulated with poly(lactide-coglycolide) to form K5 nanoparticles (K5-NP). Expression of K5 was determined by Western blot analysis and immunohistochemistry, and retinal vascular leakage was measured by permeability assay. Retinal neovascularization was evaluated using fluorescein-angiography and counting preretinal vascular cells in rats with oxygen-induced retinopathy. Effects of K5-NP on retinal inflammation were evaluated in streptozotocin-induced diabetic rats by leukostasis assay and Western blot analysis of intracellular adhesion molecule and vascular endothelial growth factor. Possible toxicities of K5-NP were evaluated using histology examination, retinal thickness measurement, and electroretinogram recording.
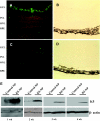
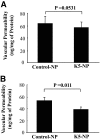
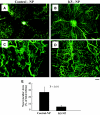
Results: K5-NP mediated efficient expression of K5 and specifically inhibited growth of endothelial cells. An intravitreal injection of K5-NP resulted in high-level expression of K5 in the inner retina of rats during the 4 weeks they were analyzed. Injection of K5-NP significantly reduced retinal vascular leakage and attenuated retinal neovascularization, when compared with the contralateral eyes injected with Control-NP in oxygen-induced retinopathy rats. K5-NP attenuated vascular endothelial growth factor and intracellular adhesion molecule-1 overexpression and reduced leukostasis and vascular leakage for at least 4 weeks after a single injection in the retina of streptozotocin-induced diabetic rats. No toxicities of K5-NP were detected to retinal structure and function.
Conclusions: K5-NP mediates efficient and sustained K5 expression in the retina and has therapeutic potential for diabetic retinopathy.
Figures

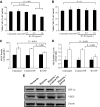
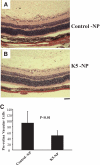
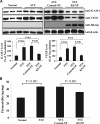
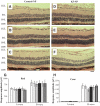
 , normal + control-NP;
, normal + control-NP;  , normal + K5-NP; ■, STZ; ▧, STZ + control-NP; ▩, STZ + K5-NP. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. (A high-quality digital representation of this figure is available in the online issue.)
, normal + K5-NP; ■, STZ; ▧, STZ + control-NP; ▩, STZ + K5-NP. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. (A high-quality digital representation of this figure is available in the online issue.)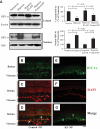
Similar articles
-
Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor.Invest Ophthalmol Vis Sci. 2011 Aug 5;52(9):6230-7. doi: 10.1167/iovs.10-6229. Invest Ophthalmol Vis Sci. 2011. PMID: 21357401 Free PMC article.
-
Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes.Diabetologia. 2004 Jan;47(1):124-31. doi: 10.1007/s00125-003-1276-4. Epub 2003 Dec 10. Diabetologia. 2004. PMID: 14666366
-
Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration.Mol Pharm. 2019 May 6;16(5):1958-1970. doi: 10.1021/acs.molpharmaceut.8b01319. Epub 2019 Mar 26. Mol Pharm. 2019. PMID: 30912953 Free PMC article.
-
Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy.Curr Issues Mol Biol. 2025 Jan 4;47(1):28. doi: 10.3390/cimb47010028. Curr Issues Mol Biol. 2025. PMID: 39852143 Free PMC article. Review.
-
Revolutionizing Retinal Therapy: The Role of Nanoparticle Drug Carriers in Managing Vascular Retinal Disorders.Clin Ophthalmol. 2025 May 15;19:1565-1582. doi: 10.2147/OPTH.S503273. eCollection 2025. Clin Ophthalmol. 2025. PMID: 40391093 Free PMC article. Review.
Cited by
-
Nanoparticles in the ocular drug delivery.Int J Ophthalmol. 2013 Jun 18;6(3):390-6. doi: 10.3980/j.issn.2222-3959.2013.03.25. Print 2013. Int J Ophthalmol. 2013. PMID: 23826539 Free PMC article.
-
Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review.Biomedicines. 2024 Mar 27;12(4):747. doi: 10.3390/biomedicines12040747. Biomedicines. 2024. PMID: 38672103 Free PMC article. Review.
-
Impacts of hypoxia-inducible factor-1 knockout in the retinal pigment epithelium on choroidal neovascularization.Invest Ophthalmol Vis Sci. 2012 Sep 14;53(10):6197-206. doi: 10.1167/iovs.11-8936. Invest Ophthalmol Vis Sci. 2012. PMID: 22915031 Free PMC article.
-
The mechanism of CCN1-enhanced retinal neovascularization in oxygen-induced retinopathy through PI3K/Akt-VEGF signaling pathway.Drug Des Devel Ther. 2015 Apr 30;9:2463-73. doi: 10.2147/DDDT.S79782. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25995618 Free PMC article.
-
Protective effects of fufang xueshuantong on diabetic retinopathy in rats.Evid Based Complement Alternat Med. 2013;2013:408268. doi: 10.1155/2013/408268. Epub 2013 Sep 24. Evid Based Complement Alternat Med. 2013. PMID: 24204392 Free PMC article.
References
-
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R: Diabetic retinopathy. Diabetes Care 1998; 21: 143– 156 - PubMed
-
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW: Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 2002; 2( Suppl.): S253– S262 - PubMed
-
- Smith LE: Pathogenesis of retinopathy of prematurity. Semin Neonatol 2003; 8: 469– 473 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

