Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation
- PMID: 19491247
- PMCID: PMC2742280
- DOI: 10.1113/jphysiol.2008.167502
Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation
Abstract
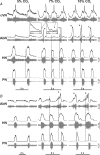
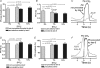
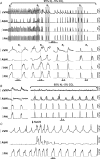
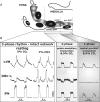
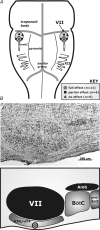
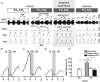
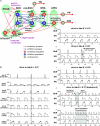
We studied respiratory neural activity generated during expiration. Motoneuronal activity was recorded simultaneously from abdominal (AbN), phrenic (PN), hypoglossal (HN) and central vagus nerves from neonatal and juvenile rats in situ. During eupnoeic activity, low-amplitude post-inspiratory (post-I) discharge was only present in AbN motor outflow. Expression of AbN late-expiratory (late-E) activity, preceding PN bursts, occurred during hypercapnia. Biphasic expiratory (biphasic-E) activity with pre-inspiratory (pre-I) and post-I discharges occurred only during eucapnic anoxia or hypercapnic anoxia. Late-E activity generated during hypercapnia (7-10% CO(2)) was abolished with pontine transections or chemical suppression of retrotrapezoid nucleus/ventrolateral parafacial (RTN/vlPF). AbN late-E activity during hypercapnia is coupled with augmented pre-I discharge in HN, truncated PN burst, and was quiescent during inspiration. Our data suggest that the pons provides a necessary excitatory drive to an additional neural oscillatory mechanism that is only activated under conditions of high respiratory drive to generate late-E activity destined for AbN motoneurones. This mechanism may arise from neurons located in the RTN/vlPF or the latter may relay late-E activity generated elsewhere. We hypothesize that this oscillatory mechanism is not a necessary component of the respiratory central pattern generator but constitutes a defensive mechanism activated under critical metabolic conditions to provide forced expiration and reduced upper airway resistance simultaneously. Possible interactions of this oscillator with components of the brainstem respiratory network are discussed.
Figures
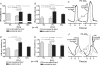


References
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem–spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. - PubMed
-
- Ballanyi K, Volker A, Richter DW. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. Neuroreport. 1994;6:165–168. - PubMed
-
- Cohen MI. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979;59:1105–1173. - PubMed
-
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol Lung Cell Mol Physiol. 1990;258:L33–L44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

