Loss of retinal cadherin facilitates mammary tumor progression and metastasis
- PMID: 19491271
- PMCID: PMC4382672
- DOI: 10.1158/0008-5472.CAN-08-4007
Loss of retinal cadherin facilitates mammary tumor progression and metastasis
Abstract
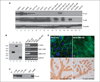
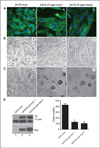
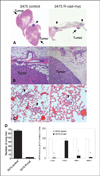
The mammary epithelium is thought to be stabilized by cell-cell adhesion mediated mainly by E-cadherin (E-cad). Here, we show that another cadherin, retinal cadherin (R-cad), is critical for maintenance of the epithelial phenotype. R-cad is expressed in nontransformed mammary epithelium but absent from tumorigenic cell lines. In vivo, R-cad was prominently expressed in the epithelium of both ducts and lobules. In human breast cancer, R-cad was down-regulated with tumor progression, with high expression in ductal carcinoma in situ and reduced expression in invasive duct carcinomas. By comparison, E-cad expression persisted in invasive breast tumors and cell lines where R-cad was lost. Consistent with these findings, R-cad knockdown in normal mammary epithelium stimulated invasiveness and disrupted formation of acini despite continued E-cad expression. Conversely, R-cad overexpression in aggressive cell lines induced glandular morphogenesis and inhibited invasiveness, tumor formation, and lung colonization. R-cad also suppressed the matrix metalloproteinase 1 (MMP1), MMP2, and cyclooxygenase 2 gene expression associated with pulmonary metastasis. The data suggest that R-cad is an adhesion molecule of the mammary epithelium, which acts as a critical regulator of the normal phenotype. As a result, R-cad loss contributes to epithelial suppression and metastatic progression.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



References
-
- Fraga MF, Herranz M, Espada J, et al. A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer Res. 2004;64:5527–5534. - PubMed
-
- Bracke M, Roy FV, Mareel M. The E-cadherin/catenin complex in invasion and metastasis. In: Gunthert U, Birchmeier W, editors. Attempts to Understand Metastasis Formation I Metastasis-related molecules. Berlin: Springer; 1996. pp. 123–161.
-
- Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. - PubMed
-
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

