White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models
- PMID: 19491274
- PMCID: PMC3857703
- DOI: 10.1158/0008-5472.CAN-08-3444
White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models
Abstract
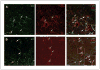
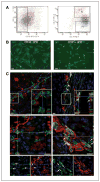
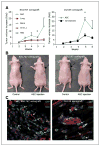
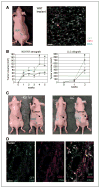
The connection between obesity and accelerated cancer progression has been established, but the mediating mechanisms are not well understood. We have shown that stromal cells from white adipose tissue (WAT) cooperate with the endothelium to promote blood vessel formation through the secretion of soluble trophic factors. Here, we hypothesize that WAT directly mediates cancer progression by serving as a source of cells that migrate to tumors and promote neovascularization. To test this hypothesis, we have evaluated the recruitment of WAT-derived cells by tumors and the effect of their engraftment on tumor growth by integrating a transgenic mouse strain engineered for expansion of traceable cells with established allograft and xenograft cancer models. Our studies show that entry of adipose stromal and endothelial cells into systemic circulation leads to their homing to and engraftment into tumor stroma and vasculature, respectively. We show that recruitment of adipose stromal cells by tumors is sufficient to promote tumor growth. Finally, we show that migration of stromal and vascular progenitor cells from WAT grafts to tumors is also associated with acceleration of cancer progression. These results provide a biological insight for the clinical association between obesity and cancer, thus outlining potential avenues for preventive and therapeutic strategies.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. - PubMed
-
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. - PubMed
-
- Flegal KM, Graubard BI, Williamson DF, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. - PubMed
-
- Rehman J, Considine RV, Bovenkerk JE, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

