Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats
- PMID: 19491293
- PMCID: PMC2724117
- DOI: 10.1152/ajpendo.90424.2008
Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats
Abstract
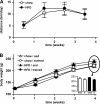
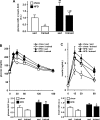
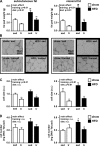
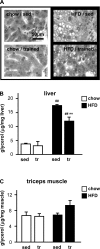
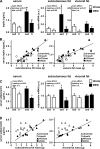
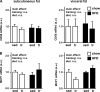
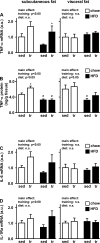
Regular physical activity improves glucose tolerance and decreases adiposity. Our aim was to investigate the effects of exercise training on subcutaneous (inguinal) and visceral (parametrial) adipose tissue in rats that were fed a chow diet (13% fat) or made insulin resistant by a high-fat diet (60% fat). Sprague-Dawley rats performed 4 wk of voluntary wheel running or were kept as sedentary controls. The training groups fed chow and the high-fat diet achieved similar running distances (8.8 +/- 1.8 and 9.3 +/- 1.9 km/day, respectively). Training improved oral glucose tolerance in chow-fed rats and prevented the glucose intolerance that occurred in sedentary rats fed the high-fat diet. In both subcutaneous and visceral adipose tissue, the high-fat diet-induced increases in fat pad weight (67% and 133%, respectively), adipocyte size (20% and 43%), and cell number (36% and 65%) were completely prevented by exercise training. Cytokine mRNA expression in visceral fat did not change with exercise training. However, in subcutaneous fat, training actually increased mRNA expression of several cytokines [IL-6: 80% (P < 0.05); TNF-alpha: 100% (P < 0.05); IL-1 receptor antagonist (IL-1Ra): 57% (P = 0.08)] with no detectable increases in serum cytokine concentrations. In summary, exercise training can overcome high-fat diet-induced impairments in glucose tolerance and increases in adipocyte size, cell number, and fat pad mass. Improved glucose tolerance was accompanied by an increase in cytokine gene expression in subcutaneous fat. This finding raises the possibility of a specific role of subcutaneous adipose tissue in adaptive responses to exercise training.
Figures
References
-
- Atzmon G, Yang XM, Muzumdar R, Ma XH, Gabriely I, Barzilai N. Differential gene expression between visceral and subcutaneous fat depots. Horm Metab Res 34: 622–628, 2002. - PubMed
-
- Chennaoui M, Drogou C, Gomez-Merino D. Effects of physical training on IL-1beta, IL-6 and IL-1ra concentrations in various brain areas of the rat. Eur Cytokine Netw 19: 8–14, 2008. - PubMed
-
- Cortez MY, Torgan CE, Brozinick JT Jr, Ivy JL. Insulin resistance of obese Zucker rats exercise trained at two different intensities. Am J Physiol Endocrinol Metab 261: E613–E619, 1991. - PubMed
-
- Craig BW, Hammons GT, Garthwaite SM, Jarett L, Holloszy JO. Adaptation of fat cells to exercise: response of glucose uptake and oxidation to insulin. J Appl Physiol 51: 1500–1506, 1981. - PubMed
-
- Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab 287: E182–E187, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

