Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease
- PMID: 19491393
- PMCID: PMC2716024
- DOI: 10.1182/blood-2009-01-197178
Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease
Abstract
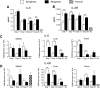
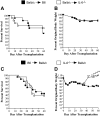
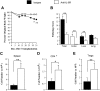
Graft-versus-host disease (GVHD) is the major complication after allogeneic bone marrow transplantation and is characterized by the overproduction of proinflammatory cytokines. In this study, we have identified interleukin-6 (IL-6) as a critical inflammatory cytokine that alters the balance between the effector and regulatory arms of the immune system and drives a proinflammatory phenotype that is a defining characteristic of GVHD. Our results demonstrate that inhibition of the IL-6 signaling pathway by way of antibody-mediated blockade of the IL-6 receptor (IL-6R) markedly reduces pathologic damage attributable to GVHD. This is accompanied by a significant increase in the absolute number of regulatory T cells (Tregs) that is due to augmentation of thymic-dependent and thymic-independent Treg production. Correspondingly, there is a significant reduction in the number of T helper 1 and T helper 17 cells in GVHD target organs, demonstrating that blockade of IL-6 signaling decreases the ratio of proinflammatory T cells to Tregs. These studies demonstrate that antibody blockade of the IL-6R serves to recalibrate the effector and regulatory arms of the immune system and represents a novel, potentially clinically translatable, strategy for the attenuation of GVHD.
Figures



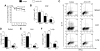
References
-
- Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft versus host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. - PubMed
-
- Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft versus host disease in H-2 incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. - PubMed
-
- Ferrara JL, Reddy P. Pathophysiology of graft versus shot disease. Semin Hematol. 2006;43:3–10. - PubMed
-
- Teshima T, Ordemann R, Reddy P, et al. Acute graft versus host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

