Effect of Ca(v)beta subunits on structural organization of Ca(v)1.2 calcium channels
- PMID: 19492014
- PMCID: PMC2688388
- DOI: 10.1371/journal.pone.0005587
Effect of Ca(v)beta subunits on structural organization of Ca(v)1.2 calcium channels
Abstract
Background: Voltage-gated Ca(v)1.2 calcium channels play a crucial role in Ca(2+) signaling. The pore-forming alpha(1C) subunit is regulated by accessory Ca(v)beta subunits, cytoplasmic proteins of various size encoded by four different genes (Ca(v)beta(1)-beta(4)) and expressed in a tissue-specific manner.
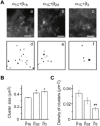
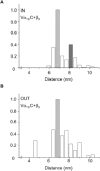
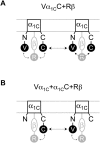
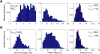
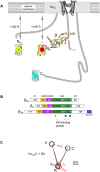
Methods and results: Here we investigated the effect of three major Ca(v)beta types, beta(1b), beta(2d) and beta(3), on the structure of Ca(v)1.2 in the plasma membrane of live cells. Total internal reflection fluorescence microscopy showed that the tendency of Ca(v)1.2 to form clusters depends on the type of the Ca(v)beta subunit present. The highest density of Ca(v)1.2 clusters in the plasma membrane and the smallest cluster size were observed with neuronal/cardiac beta(1b) present. Ca(v)1.2 channels containing beta(3), the predominant Ca(v)beta subunit of vascular smooth muscle cells, were organized in a significantly smaller number of larger clusters. The inter- and intramolecular distances between alpha(1C) and Ca(v)beta in the plasma membrane of live cells were measured by three-color FRET microscopy. The results confirm that the proximity of Ca(v)1.2 channels in the plasma membrane depends on the Ca(v)beta type. The presence of different Ca(v)beta subunits does not result in significant differences in the intramolecular distance between the termini of alpha(1C), but significantly affects the distance between the termini of neighbor alpha(1C) subunits, which varies from 67 A with beta(1b) to 79 A with beta(3).
Conclusions: Thus, our results show that the structural organization of Ca(v)1.2 channels in the plasma membrane depends on the type of Ca(v)beta subunits present.
Conflict of interest statement
Figures
References
-
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- Silver RA, Lamb AG, Bolsover SR. Calcium hotspots caused by L-channel clustering promote morphological changes in neuronal growth cones. Nature. 1990;343:751–754. - PubMed
-
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. - PubMed
-
- Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann NY Acad Sci. 1998;853:20–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

