OPTICAL PHASE CONJUGATION FOR TURBIDITY SUPPRESSION IN BIOLOGICAL SAMPLES
- PMID: 19492016
- PMCID: PMC2688902
- DOI: 10.1038/nphoton.2007.297
OPTICAL PHASE CONJUGATION FOR TURBIDITY SUPPRESSION IN BIOLOGICAL SAMPLES
Abstract
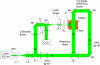
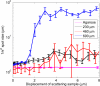
Elastic optical scattering, the dominant light interaction process in biological tissues, prevents tissues from being transparent. While scattering may appear stochastic, it is in fact deterministic in nature. We show that, despite experimental imperfections, optical phase conjugation (lambda = 532 nm) can force a transmitted light field to retrace its trajectory through a biological target and recover the original light field. For a 0.69 mm thick chicken breast tissue section, we can enhance point source light return by approximately 5x10(3) times and achieve a light transmission enhancement factor of 3.8 within a collection angle of 29 degrees . Additionally, we find that the reconstruction's quality, measured by the width of the reconstructed point source, is independent of tissue thickness (up to 0.69 mm thick). This phenomenon may be used to enhance light transmission through tissue, enable measurement of small tissue movements, and form the basis of new tissue imaging techniques.
Figures









References
-
- Cheong WF, Prahl SA, Welch AJ. A review of the optical-properties of biological tissues. IEEE J. Quantum Electron. 1990;26:2166–2185.
-
- Leith EN, Upatneiks J. Holographic imagery through diffusing media. J. Opt. Soc. Am. 1966;56:523.
-
- Levenson MD. High-resolution imaging by wave-front conjugation. Opt. Lett. 1980;5:182–184. - PubMed
-
- McFarlane RA, Steel DG. Laser-oscillator using resonator with self-pumped phase-conjugate mirror. Opt. Lett. 1983;8:208–210. - PubMed
-
- Gower MC. Krf laser-amplifier with phase-conjugate Brillouin retro-reflectors. Opt. Lett. 1982;7:423–425. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
