SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress
- PMID: 19492079
- PMCID: PMC2683566
- DOI: 10.1371/journal.pone.0005684
SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress
Abstract
Background: Ion transporters of the Slc30A- (ZnT-) family regulate zinc fluxes into sub-cellular compartments. beta-cells depend on zinc for both insulin crystallization and regulation of cell mass.
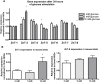
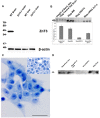
Methodology/principal findings: This study examined: the effect of glucose and zinc chelation on ZnT gene and protein levels and apoptosis in beta-cells and pancreatic islets, the effects of ZnT-3 knock-down on insulin secretion in a beta-cell line and ZnT-3 knock-out on glucose metabolism in mice during streptozotocin-induced beta-cell stress. In INS-1E cells 2 mM glucose down-regulated ZnT-3 and up-regulated ZnT-5 expression relative to 5 mM. 16 mM glucose increased ZnT-3 and decreased ZnT-8 expression. Zinc chelation by DEDTC lowered INS-1E insulin content and insulin expression. Furthermore, zinc depletion increased ZnT-3- and decreased ZnT-8 gene expression whereas the amount of ZnT-3 protein in the cells was decreased. Zinc depletion and high glucose induced apoptosis and necrosis in INS-1E cells. The most responsive zinc transporter, ZnT-3, was investigated further; by immunohistochemistry and western blotting ZnT-3 was demonstrated in INS-1E cells. 44% knock-down of ZnT-3 by siRNA transfection in INS-1E cells decreased insulin expression and secretion. Streptozotocin-treated mice had higher glucose levels after ZnT-3 knock-out, particularly in overt diabetic animals.
Conclusion/significance: Zinc transporting proteins in beta-cells respond to variations in glucose and zinc levels. ZnT-3, which is pivotal in the development of cellular changes as also seen in type 2 diabetes (e.g. amyloidosis in Alzheimer's disease) but not previously described in beta-cells, is present in this cell type, up-regulated by glucose in a concentration dependent manner and up-regulated by zinc depletion which by contrast decreased ZnT-3 protein levels. Knock-down of the ZnT-3 gene lowers insulin secretion in vitro and affects in vivo glucose metabolism after streptozotocin treatment.
Conflict of interest statement
Figures

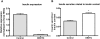




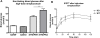
References
-
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. - PubMed
-
- Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–115. - PubMed
-
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. - PubMed
-
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. - PubMed
-
- Chang I, Cho N, Koh JY, Lee MS. Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia. 2003;46:1220–1227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

