Vibriobactin antibodies: a vaccine strategy
- PMID: 19492834
- PMCID: PMC2951131
- DOI: 10.1021/jm900119q
Vibriobactin antibodies: a vaccine strategy
Abstract
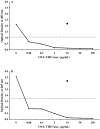
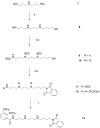
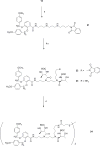
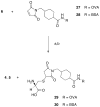
A new target strategy in the development of bacterial vaccines, the induction of antibodies to microbial outer membrane ferrisiderophore complexes, is explored. A vibriobactin (VIB) analogue, with a thiol tether, 1-(2,3-dihydroxybenzoyl)-5,9-bis[[(4S,5R)-2-(2,3-dihydroxyphenyl)-4,5-dihydro-5-methyl-4-oxazolyl]carbonyl]-14-(3-mercaptopropanoyl)-1,5,9,14-tetraazatetradecane, was synthesized and linked to ovalbumin (OVA) and bovine serum albumin (BSA). The antigenicity of the VIB microbial iron chelator conjugates and their iron complexes was evaluated. When mice were immunized with the resulting OVA-VIB conjugate, a selective and unequivocal antigenic response to the VIB hapten was observed; IgG monoclonal antibodies specific to the vibriobactin fragment of the BSA and OVA conjugates were isolated. The results are consistent with the idea that the isolated adducts of siderophores covalently linked to their bacterial outer membrane receptors represent a credible target for vaccine development.
Figures






Similar articles
-
Unique iron coordination in iron-chelating molecule vibriobactin helps Vibrio cholerae evade mammalian siderocalin-mediated immune response.J Biol Chem. 2012 Mar 16;287(12):8912-9. doi: 10.1074/jbc.M111.316034. Epub 2012 Jan 30. J Biol Chem. 2012. PMID: 22291019 Free PMC article.
-
Catechol Siderophore Transport by Vibrio cholerae.J Bacteriol. 2015 Sep;197(17):2840-9. doi: 10.1128/JB.00417-15. Epub 2015 Jun 22. J Bacteriol. 2015. PMID: 26100039 Free PMC article.
-
Monoclonal antibodies to outer membrane antigens of Vibrio cholerae.Infect Immun. 1985 Jul;49(1):122-31. doi: 10.1128/iai.49.1.122-131.1985. Infect Immun. 1985. PMID: 3159676 Free PMC article.
-
Siderocalin outwits the coordination chemistry of vibriobactin, a siderophore of Vibrio cholerae.ACS Chem Biol. 2013 Sep 20;8(9):1882-7. doi: 10.1021/cb4002552. Epub 2013 Jun 18. ACS Chem Biol. 2013. PMID: 23755875 Free PMC article.
-
Iron acquisition in Vibrio cholerae.Biometals. 2007 Jun;20(3-4):405-16. doi: 10.1007/s10534-006-9073-4. Epub 2007 Jan 10. Biometals. 2007. PMID: 17216354 Review.
Cited by
-
Substituent effects on desferrithiocin and desferrithiocin analogue iron-clearing and toxicity profiles.J Med Chem. 2012 Aug 23;55(16):7090-103. doi: 10.1021/jm300509y. Epub 2012 Aug 13. J Med Chem. 2012. PMID: 22889170 Free PMC article.
-
Siderophore-based immunization strategy to inhibit growth of enteric pathogens.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):13462-13467. doi: 10.1073/pnas.1606290113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821741 Free PMC article.
-
Engineering Siderophore Biosynthesis and Regulation Pathways to Increase Diversity and Availability.Biomolecules. 2023 Jun 7;13(6):959. doi: 10.3390/biom13060959. Biomolecules. 2023. PMID: 37371539 Free PMC article. Review.
-
Advancing the use of Lactobacillus acidophilus surface layer protein A for the treatment of intestinal disorders in humans.Gut Microbes. 2015;6(6):392-7. doi: 10.1080/19490976.2015.1107697. Gut Microbes. 2015. PMID: 26647142 Free PMC article.
-
Harnessing Iron Acquisition Machinery to Target Enterobacteriaceae.J Infect Dis. 2021 Jun 16;223(12 Suppl 2):S307-S313. doi: 10.1093/infdis/jiaa440. J Infect Dis. 2021. PMID: 33330928 Free PMC article. Review.
References
-
- Bergeron RJ, Brittenham GM. The Development of Iron Chelators for Clinical Use. CRC; Boca Raton, Fl: 1994.
-
- Bergeron RJ, McManis JS, Wiegand J, Weimar WR. A Search for Clinically Effective Iron Chelators. In: Badman DG, Bergeron RJ, Brittenham GM, editors. Iron Chelators: New Development Strategies. Saratoga; Ponte Vedra Beach, FL: 2000. pp. 253–292.
-
- Crichton RR. Inorganic Biochemistry of Iron Metabolism. J. Wiley & Sons; Chichester, UK: 2001.
-
- Chipperfield JR, Ratledge C. Salicylate Is Not a Bacterial Siderophore: A Theoretical Study. BioMetals. 2000;13:165–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

