An in vivo covalent TMP-tag based on proximity-induced reactivity
- PMID: 19492849
- PMCID: PMC2796251
- DOI: 10.1021/cb900062k
An in vivo covalent TMP-tag based on proximity-induced reactivity
Abstract
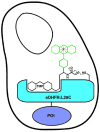
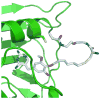
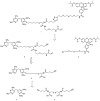
Chemical tags for live cell imaging are emerging as viable alternatives to the fluorescent proteins for labeling proteins with small molecule probes. Among reported chemical tags, trimethoprim (TMP)-tag stands out for having sufficient cell permeability and selectivity to allow imaging of intracellular proteins. TMP-tag provides a noncovalent label in which the protein of interest is fused to E. coli dihydrofolate reductase (DHFR) and then labeled with a cell-permeable TMP-probe heterodimer. To complement the utility of the noncovalent TMP-tag, we sought to render the TMP-tag covalent for applications such as single-molecule tracking and pulse-chase labeling that would benefit from a more permanent modification. On the basis of the long-standing use of proximity-induced reactivity for irreversible inhibitor design and its more recent application to in vitro chemical biology tools, we designed an eDHFR variant with a unique cysteine residue positioned to react with an acrylamide electrophile installed on the TMP-probe label. In vitro experiments show that the eDHFR:L28C nucleophile reacts rapidly and quantitatively with the TMP-acrylamide-probe. Most significantly, the balance in reactivity provided by the acrylamide electrophile allows intracellular proteins tagged with eDHFR:L28C to be labeled with a TMP-acrylamide-fluorescein heterotrimer in live cells with minimal background. Thus, the TMP electrophile described here can be used immediately as a covalent chemical tag in live cells. Moreover, proximity-induced reactivity is shown to be sufficiently selective for use in a living cell, suggesting a general approach for the development of orthogonal covalent chemical tags from existing noncovalent ligand-protein pairs.
Figures


References
-
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Review - The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
-
- Tsien RY. The green fluorescent protein. Annual Review of Biochemistry. 1998;67:509–544. - PubMed
-
- Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. - PubMed
-
- O'Hare HM, Johnsson K, Gautier A. Chemical probes shed light on protein function. Curr Opin Struct Biol. 2007;17:488–494. - PubMed
-
- Miller LW, Cornish VW. Selective chemical labeling of proteins in living cells. Curr Opin Chem Biol. 2005;9:56–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

