Mechanisms of strand break formation in DNA due to the direct effect of ionizing radiation: the dependency of free base release on the length of alternating CG oligodeoxynucleotides
- PMID: 19492855
- PMCID: PMC2791348
- DOI: 10.1021/jp900803b
Mechanisms of strand break formation in DNA due to the direct effect of ionizing radiation: the dependency of free base release on the length of alternating CG oligodeoxynucleotides
Abstract
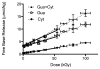
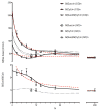
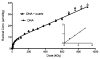
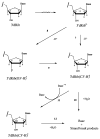
The question of how NA base sequence influences the yield of DNA strand breaks produced by the direct effect of ionizing radiation was investigated in a series of oligodeoxynucleotides of the form (d(CG)(n))(2) and (d(GC)(n))(2). The yields of free base release from X-irradiated DNA films containing 2.5 waters/nucleotide were measured by HPLC as a function of oligomer length. For (d(CG)(n))(2), the ratio of the Gua yield to Cyt yield, R, was relatively constant at 2.4-2.5 for n = 2-4 and it decreased to 1.2 as n increased from 5 to 10. When Gua was moved to the 5' end, for example going from d(CG)(5) to d(GC)(5), R dropped from 1.9 +/- 0.1 to 1.1 +/- 0.1. These effects are poorly described if the chemistry at the oligomer ends is assumed to be independent of the remainder of the oligomer. A mathematical model incorporating charge transfer through the base stack was derived to explain these effects. In addition, EPR was used to measure the yield of trapped-deoxyribose radicals at 4 K following X-irradiation at 4 K. The yield of free base release was substantially greater, by 50-100 nmol/J, than the yield of trapped-deoxyribose radicals. Therefore, a large fraction of free base release stems from a nonradical intermediate. For this intermediate, a deoxyribose carbocation formed by two one-electron oxidations is proposed. This reaction pathway requires that the hole (electron loss site) transfers through the base stack and, upon encountering a deoxyribose hole, oxidizes that site to form a deoxyribose carbocation. This reaction mechanism provides a consistent way of explaining both the absence of trapped radical intermediates and the unusual dependence of free base release on oligomer length.
Figures


References
-
- von Sonntag C. The Chemical Basis of Radiation Biology. Taylor and Francis; New York: 1987.
-
- von Sonntag C. Free-Radical-Induced DNA Damage. Springer-Verlag; Germany: 2006.
-
- Krisch RE, Flick MB, Trumbore CN. Radiat Res. 1991;126:251. - PubMed
-
- Milligan JR, Aguilera JA, Ward JF. Radiat Res. 1993;133:158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

