The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma
- PMID: 19493313
- PMCID: PMC3610526
- DOI: 10.1111/j.1755-148X.2009.00585.x
The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma
Abstract
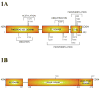
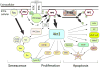
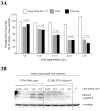
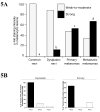
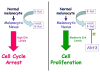
Melanocytes undergo extensive genetic changes during transformation into aggressive melanomas. These changes deregulate genes whose aberrant activity promotes the development of this disease. The phosphoinositide-3-kinase (PI3K) and mitogen-activated protein (MAP) kinase pathways are two key signaling cascades that have been found to play prominent roles in melanoma development. These pathways relay extra-cellular signals via an ordered series of consecutive phosphorylation events from cell surface throughout the cytoplasm and nucleus regulating diverse cellular processes including proliferation, survival, invasion and angiogenesis. It is generally accepted that therapeutic agents would need to target these two pathways to be an effective therapy for the long-term treatment of advanced-stage melanoma patients. This review provides an overview of the PI3 kinase pathway focusing specifically on two members of the pathway, called PTEN and Akt3, which play important roles in melanoma development. Mechanisms leading to deregulation of these two proteins and therapeutic implications of targeting this signaling cascade to treat melanoma are detailed in this review.
Figures




Similar articles
-
Differential AKT dependency displayed by mouse models of BRAFV600E-initiated melanoma.J Clin Invest. 2013 Dec;123(12):5104-18. doi: 10.1172/JCI69619. Epub 2013 Nov 8. J Clin Invest. 2013. PMID: 24200692 Free PMC article.
-
In vitro treatment of melanoma brain metastasis by simultaneously targeting the MAPK and PI3K signaling pathways.Int J Mol Sci. 2014 May 16;15(5):8773-94. doi: 10.3390/ijms15058773. Int J Mol Sci. 2014. PMID: 24840574 Free PMC article.
-
Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance.Adv Enzyme Regul. 2006;46:249-79. doi: 10.1016/j.advenzreg.2006.01.004. Epub 2006 Jul 18. Adv Enzyme Regul. 2006. PMID: 16854453
-
Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway.Leukemia. 2011 Jul;25(7):1064-79. doi: 10.1038/leu.2011.46. Epub 2011 Mar 25. Leukemia. 2011. PMID: 21436840 Review.
-
Functional and therapeutic significance of Akt deregulation in malignant melanoma.Cancer Metastasis Rev. 2005 Jun;24(2):273-85. doi: 10.1007/s10555-005-1577-9. Cancer Metastasis Rev. 2005. PMID: 15986137 Review.
Cited by
-
Systematic investigation into the role of intermittent high glucose in pancreatic beta-cells.Int J Clin Exp Med. 2015 Apr 15;8(4):5462-9. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26131124 Free PMC article.
-
The soy-derived peptide Lunasin inhibits invasive potential of melanoma initiating cells.Oncotarget. 2017 Apr 11;8(15):25525-25541. doi: 10.18632/oncotarget.16066. Oncotarget. 2017. PMID: 28424421 Free PMC article.
-
Deletion of PTENP1 pseudogene in human melanoma.J Invest Dermatol. 2011 Dec;131(12):2497-500. doi: 10.1038/jid.2011.232. Epub 2011 Aug 11. J Invest Dermatol. 2011. PMID: 21833010 Free PMC article. No abstract available.
-
PPAR-gamma induced AKT3 expression increases levels of mitochondrial biogenesis driving prostate cancer.Oncogene. 2021 Apr;40(13):2355-2366. doi: 10.1038/s41388-021-01707-7. Epub 2021 Mar 2. Oncogene. 2021. PMID: 33654198 Free PMC article.
-
Merlin is a negative regulator of human melanoma growth.PLoS One. 2012;7(8):e43295. doi: 10.1371/journal.pone.0043295. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912849 Free PMC article.
References
-
- Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280:35195–35202. - PubMed
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

