Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial
- PMID: 19493352
- PMCID: PMC2717450
- DOI: 10.1186/cc7903
Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial
Abstract
Introduction: The development of resistance by bacterial species is a compelling issue to reconsider indications and administration of antibiotic treatment. Adequate indications and duration of therapy are particularly important for the use of highly potent substances in the intensive care setting. Until recently, no laboratory marker has been available to differentiate bacterial infection from viral or non-infectious inflammatory reaction; however, over the past years, procalcitonin (PCT) is the first among a large array of inflammatory variables that offers this possibility. The present study aimed to investigate the clinical usefulness of PCT for guiding antibiotic therapy in surgical intensive care patients.
Methods: All patients requiring antibiotic therapy based on confirmed or highly suspected bacterial infections and at least two concomitant systemic inflammatory response syndrome criteria were eligible. Patients were randomly assigned to either a PCT-guided (study group) or a standard (control group) antibiotic regimen. Antibiotic therapy in the PCT-guided group was discontinued, if clinical signs and symptoms of infection improved and PCT decreased to <1 ng/ml or the PCT value was >1 ng/ml, but had dropped to 25 to 35% of the initial value over three days. In the control group antibiotic treatment was applied as standard regimen over eight days.
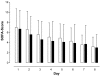
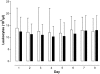
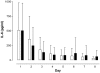
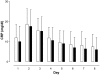
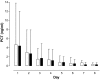
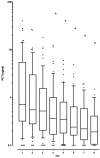
Results: A total of 110 surgical intensive care patients receiving antibiotic therapy after confirmed or high-grade suspected infections were enrolled in this study. In 57 patients antibiotic therapy was guided by daily PCT and clinical assessment and adjusted accordingly. The control group comprised 53 patients with a standardized duration of antibiotic therapy over eight days. Demographic and clinical data were comparable in both groups. However, in the PCT group the duration of antibiotic therapy was significantly shorter than compared to controls (5.9 +/- 1.7 versus 7.9 +/- 0.5 days, P < 0.001) without negative effects on clinical outcome.
Conclusions: Monitoring of PCT is a helpful tool for guiding antibiotic treatment in surgical intensive care patients. This may contribute to an optimized antibiotic regimen with beneficial effects on microbial resistance and costs in intensive care medicine. ANNOTATION: Results were previously published in German in Anaesthesist 2008; 57: 571-577 (PMID: 18463831).
Trial registration: ISRCTN10288268.
Figures
Comment in
-
When once is not enough--further evidence of procalcitonin-guided antibiotic stewardship.Crit Care. 2009;13(4):165. doi: 10.1186/cc7935. Epub 2009 Jul 13. Crit Care. 2009. PMID: 19664168 Free PMC article.
-
Procalcitonin to guide duration of antibiotic therapy in intensive care patients: some research questions.Crit Care. 2009;13(4):414; author reply 414. doi: 10.1186/cc7958. Epub 2009 Jul 30. Crit Care. 2009. PMID: 19664199 Free PMC article. No abstract available.
References
-
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. - DOI - PubMed
-
- ACCP/SCCM Consensus Conference Committee Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

