Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency
- PMID: 19493963
- PMCID: PMC2724260
- DOI: 10.1152/ajprenal.90765.2008
Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency
Abstract
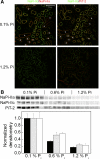
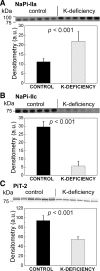
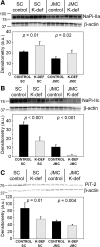
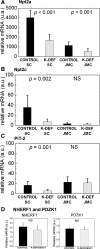
Dietary potassium (K) deficiency is accompanied by phosphaturia and decreased renal brush border membrane (BBM) vesicle sodium (Na)-dependent phosphate (P(i)) transport activity. Our laboratory previously showed that K deficiency in rats leads to increased abundance in the proximal tubule BBM of the apical Na-P(i) cotransporter NaPi-IIa, but that the activity, diffusion, and clustering of NaPi-IIa could be modulated by the altered lipid composition of the K-deficient BBM (Zajicek HK, Wang H, Puttaparthi K, Halaihel N, Markovich D, Shayman J, Beliveau R, Wilson P, Rogers T, Levi M. Kidney Int 60: 694-704, 2001; Inoue M, Digman MA, Cheng M, Breusegem SY, Halaihel N, Sorribas V, Mantulin WW, Gratton E, Barry NP, Levi M. J Biol Chem 279: 49160-49171, 2004). Here we investigated the role of the renal Na-P(i) cotransporters NaPi-IIc and PiT-2 in K deficiency. Using Western blotting, immunofluorescence, and quantitative real-time PCR, we found that, in rats and in mice, K deficiency is associated with a dramatic decrease in the NaPi-IIc protein abundance in proximal tubular BBM and in NaPi-IIc mRNA. In addition, we documented the presence of a third Na-coupled P(i) transporter in the renal BBM, PiT-2, whose abundance is also decreased by dietary K deficiency in rats and in mice. Finally, electron microscopy showed subcellular redistribution of NaPi-IIc in K deficiency: in control rats, NaPi-IIc immunolabel was primarily in BBM microvilli, whereas, in K-deficient rats, NaPi-IIc BBM label was reduced, and immunolabel was prevalent in cytoplasmic vesicles. In summary, our results demonstrate that decreases in BBM abundance of the phosphate transporter NaPi-IIc and also PiT-2 might contribute to the phosphaturia of dietary K deficiency, and that the three renal BBM phosphate transporters characterized so far can be differentially regulated by dietary perturbations.
Figures





Similar articles
-
Renal phosphaturia during metabolic acidosis revisited: molecular mechanisms for decreased renal phosphate reabsorption.Pflugers Arch. 2008 Nov;457(2):539-49. doi: 10.1007/s00424-008-0530-5. Epub 2008 Jun 6. Pflugers Arch. 2008. PMID: 18535837
-
Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters.Pflugers Arch. 2010 Aug;460(3):677-87. doi: 10.1007/s00424-010-0841-1. Epub 2010 Jun 5. Pflugers Arch. 2010. PMID: 20526720
-
Magnesium stimulates renal phosphate reabsorption.Am J Physiol Renal Physiol. 2008 Oct;295(4):F1126-33. doi: 10.1152/ajprenal.00353.2007. Epub 2008 Aug 13. Am J Physiol Renal Physiol. 2008. PMID: 18701629
-
Updates on renal phosphate transport.Curr Opin Nephrol Hypertens. 2025 Jul 1;34(4):269-275. doi: 10.1097/MNH.0000000000001090. Epub 2025 May 13. Curr Opin Nephrol Hypertens. 2025. PMID: 40357590 Free PMC article. Review.
-
Renal phosphate handling and inherited disorders of phosphate reabsorption: an update.Pediatr Nephrol. 2019 Apr;34(4):549-559. doi: 10.1007/s00467-017-3873-3. Epub 2017 Dec 23. Pediatr Nephrol. 2019. PMID: 29275531 Review.
Cited by
-
Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi).Pflugers Arch. 2019 Jan;471(1):83-98. doi: 10.1007/s00424-018-2231-z. Epub 2018 Nov 5. Pflugers Arch. 2019. PMID: 30393837 Free PMC article. Review.
-
Ablation of TRPC3 compromises bicarbonate and phosphate transporter activity in mice proximal tubular cells.Clin Exp Pharmacol Physiol. 2023 Mar;50(3):247-255. doi: 10.1111/1440-1681.13741. Epub 2022 Dec 18. Clin Exp Pharmacol Physiol. 2023. PMID: 36433745 Free PMC article.
-
The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II.Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F343-51. doi: 10.1152/ajprenal.00247.2016. Epub 2016 Jun 8. Am J Physiol Renal Physiol. 2016. PMID: 27279487 Free PMC article.
-
Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone.Am J Physiol Cell Physiol. 2011 Oct;301(4):C850-61. doi: 10.1152/ajpcell.00412.2010. Epub 2011 May 18. Am J Physiol Cell Physiol. 2011. PMID: 21593452 Free PMC article.
-
Conferring electrogenicity to the electroneutral phosphate cotransporter NaPi-IIc (SLC34A3) reveals an internal cation release step.Pflugers Arch. 2013 Sep;465(9):1261-79. doi: 10.1007/s00424-013-1261-9. Epub 2013 Mar 21. Pflugers Arch. 2013. PMID: 23515872
References
-
- Ambuhl PM, Zajicek HK, Wang H, Puttaparthi K, Levi M. Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int 53: 1288–1298, 1998. - PubMed
-
- Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006. - PMC - PubMed
-
- Biber J, Custer M, Werner A, Kaissling B, Murer H. Localization of NaPi-1, a Na/Pi cotransporter, in rabbit kidney proximal tubules. II. Localization by immunohistochemistry. Pflügers Arch 424: 210–215, 1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

