Activation of the nitric oxide-cGMP pathway reduces phasic contractions in neonatal rat bladder strips via protein kinase G
- PMID: 19493964
- PMCID: PMC2724251
- DOI: 10.1152/ajprenal.00207.2009
Activation of the nitric oxide-cGMP pathway reduces phasic contractions in neonatal rat bladder strips via protein kinase G
Abstract
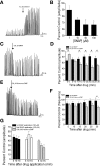
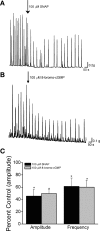
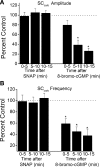
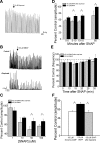
Nitric oxide (NO), a neurotransmitter in the lower urinary tract, stimulates soluble guanylyl cyclase (sGC) and in turn cGMP-dependent protein kinase G (PKG) to modulate a number of downstream targets. NO donors reduce bladder hyperactivity in some pathological models but do not affect normal bladder activity in the adult rat. In this study, the NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP; 100 microM) decreased the amplitude and frequency of spontaneous and carbachol-enhanced contractions in neonatal rat bladder strips, which are intrinsically hyperactive. This effect was blocked by inhibition of sGC and mimicked by application of a membrane-permeable cGMP analog (8-bromo-cGMP, 100 microM). Inhibition of PKG prevented or reversed the inhibitory effects of 8-bromo-cGMP. A portion of the SNAP-mediated inhibition was also dependent upon PKG; however, a short-lasting, sGC-dependent inhibitory effect of SNAP was still present after PKG inhibition. Inhibition of NO synthase with L-NAME (100 microM) did not change the amplitude or frequency of contractions. However, inhibition of endogenous phosphodiesterase (PDE)-5 with zaprinast (25 microM) reduced the amplitude and frequency of phasic contractions and increased the magnitude of inhibition produced by maximal concentrations of SNAP, suggesting that endogenous PDEs are constitutively active and regulate cGMP production. These results suggest that the NO-cGMP-PKG pathway may be involved in inhibitory control of the neonatal rat bladder.
Figures

References
-
- Andersson KE, Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol 12: 274–280, 1994. - PubMed
-
- Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl 175: 43–53, 1995. - PubMed
-
- Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

