Determinants of hepatitis C virus p7 ion channel function and drug sensitivity identified in vitro
- PMID: 19493992
- PMCID: PMC2715780
- DOI: 10.1128/JVI.00521-09
Determinants of hepatitis C virus p7 ion channel function and drug sensitivity identified in vitro
Abstract
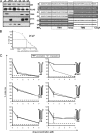
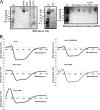
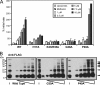
Hepatitis C virus (HCV) chronically infects 170 million individuals, causing severe liver disease. Although antiviral chemotherapy exists, the current regimen is ineffective in 50% of cases due to high levels of innate virus resistance. New, virus-specific therapies are forthcoming although their development has been slow and they are few in number, driving the search for new drug targets. The HCV p7 protein forms an ion channel in vitro and is critical for the secretion of infectious virus. p7 displays sensitivity to several classes of compounds, making it an attractive drug target. We recently demonstrated that p7 compound sensitivity varies according to viral genotype, yet little is known of the residues within p7 responsible for channel activity or drug interactions. Here, we have employed a liposome-based assay for p7 channel function to investigate the genetic basis for compound sensitivity. We demonstrate using chimeric p7 proteins that neither the two trans-membrane helices nor the p7 basic loop individually determines compound sensitivity. Using point mutation analysis, we identify amino acids important for channel function and demonstrate that null mutants exert a dominant negative effect over wild-type protein. We show that, of the three hydrophilic regions within the amino-terminal trans-membrane helix, only the conserved histidine at position 17 is important for genotype 1b p7 channel activity. Mutations predicted to play a structural role affect both channel function and oligomerization kinetics. Lastly, we identify a region at the p7 carboxy terminus which may act as a specific sensitivity determinant for the drug amantadine.
Figures




Similar articles
-
A conserved basic loop in hepatitis C virus p7 protein is required for amantadine-sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria.J Gen Virol. 2004 Feb;85(Pt 2):451-461. doi: 10.1099/vir.0.19634-0. J Gen Virol. 2004. PMID: 14769903
-
Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel.Hepatology. 2008 Dec;48(6):1779-90. doi: 10.1002/hep.22555. Hepatology. 2008. PMID: 18828153 Free PMC article.
-
Resistance mutations define specific antiviral effects for inhibitors of the hepatitis C virus p7 ion channel.Hepatology. 2011 Jul;54(1):79-90. doi: 10.1002/hep.24371. Hepatology. 2011. PMID: 21520195
-
Hepatitis C virus p7: molecular function and importance in hepatitis C virus life cycle and potential antiviral target.Liver Int. 2011 May;31(5):606-17. doi: 10.1111/j.1478-3231.2010.02442.x. Epub 2011 Jan 24. Liver Int. 2011. PMID: 21457434 Review.
-
Inhibition of HCV p7 as a therapeutic target.Curr Opin Investig Drugs. 2010 Feb;11(2):175-81. Curr Opin Investig Drugs. 2010. PMID: 20112167 Review.
Cited by
-
Targeting the Channel Activity of Viroporins.Adv Protein Chem Struct Biol. 2016;104:307-355. doi: 10.1016/bs.apcsb.2015.12.003. Epub 2016 Jan 7. Adv Protein Chem Struct Biol. 2016. PMID: 27038378 Free PMC article. Review.
-
NMR structure and ion channel activity of the p7 protein from hepatitis C virus.J Biol Chem. 2010 Oct 8;285(41):31446-61. doi: 10.1074/jbc.M110.122895. Epub 2010 Jul 28. J Biol Chem. 2010. PMID: 20667830 Free PMC article.
-
NMR studies of p7 protein from hepatitis C virus.Eur Biophys J. 2010 Jun;39(7):1097-104. doi: 10.1007/s00249-009-0533-y. Epub 2009 Sep 2. Eur Biophys J. 2010. PMID: 19727701 Free PMC article.
-
Efficiency of E2-p7 processing modulates production of infectious hepatitis C virus.J Virol. 2013 Oct;87(20):11255-66. doi: 10.1128/JVI.01807-13. Epub 2013 Aug 14. J Virol. 2013. PMID: 23946462 Free PMC article.
-
Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production.PLoS Pathog. 2010 Sep 2;6(9):e1001087. doi: 10.1371/journal.ppat.1001087. PLoS Pathog. 2010. PMID: 20824094 Free PMC article.
References
-
- Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244359-362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

