Combinations of the first and next generations of human immunodeficiency virus (HIV) fusion inhibitors exhibit a highly potent synergistic effect against enfuvirtide- sensitive and -resistant HIV type 1 strains
- PMID: 19493996
- PMCID: PMC2715752
- DOI: 10.1128/JVI.00168-09
Combinations of the first and next generations of human immunodeficiency virus (HIV) fusion inhibitors exhibit a highly potent synergistic effect against enfuvirtide- sensitive and -resistant HIV type 1 strains
Abstract
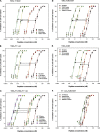
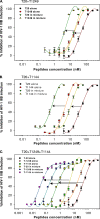
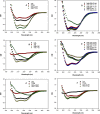
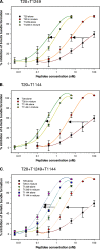
T20 (generic name, enfuvirtide; brand name, Fuzeon) is a first-generation human immunodeficiency virus (HIV) fusion inhibitor approved for salvage therapy of HIV-infected patients refractory to current antiretroviral drugs. However, its clinical use is limited because of rapid emergence of T20-resistant viruses in T20-treated patients. Therefore, T1249 and T1144 are being developed as the second- and third-generation HIV fusion inhibitors, respectively, with improved efficacy and drug resistance profiles. Here, we found that combinations of T20 with T1249 and/or T1144 resulted in exceptionally potent synergism (combination index, <0.01) against HIV-1-mediated membrane fusion by 2 to 3 orders of magnitude in dose reduction. Highly potent synergistic antiviral efficacy was also achieved against infection by laboratory-adapted and primary HIV-1 strains, including T20-resistant variants. The mechanism underlying the synergistic effect could be attributed to the fact that T20, T1249, and T1144 all contain different functional domains and have different primary binding sites in gp41. As such, they may work cooperatively to inhibit gp41 six-helix bundle core formation, thereby suppressing virus-cell fusion. Therefore, these findings strongly imply that, rather than replacing T20, combining it with HIV fusion inhibitors of different generations might produce synergistic activity against both T20-sensitive and -resistant HIV-1 strains, suggesting a new therapeutic strategy for the treatment of HIV-1 infection/AIDS.
Figures

References
-
- Bellamy-McIntyre, A. K., C. S. Lay, S. Baar, A. L. Maerz, G. H. Talbo, H. E. Drummer, and P. Poumbourios. 2007. Functional links between the fusion peptide-proximal polar segment and membrane-proximal region of human immunodeficiency virus gp41 in distinct phases of membrane fusion. J. Biol. Chem. 28223104-23116. - PubMed
-
- Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89263-273. - PubMed
-
- Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93681-684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

