A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA
- PMID: 19494001
- PMCID: PMC2715786
- DOI: 10.1128/JVI.00826-09
A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA
Abstract
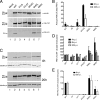
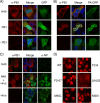
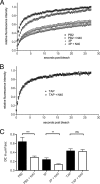
Influenza A virus segment 2 is known to encode two polypeptides in overlapping open reading frames: PB1, the polymerase, and PB1-F2, a proapoptotic virulence factor. We show that a third major polypeptide is synthesized from PB1 mRNA via differential AUG codon usage. PB1 codon 40 directs translation of an N-terminally truncated version of the polypeptide (N40) that lacks transcriptase function but nevertheless interacts with PB2 and the polymerase complex in the cellular environment. Importantly, the expression of N40, PB1-F2, and PB1 are interdependent, and certain mutations previously used to ablate PB1-F2 production affected N40 accumulation. Removal of the PB1-F2 AUG upregulated N40 synthesis, while truncating PB1-F2 after codon 8 (with a concomitant M40I change in PB1) abolished N40 expression. A virus lacking both N40 and PB1-F2 replicated normally. However, viruses that did not express N40 but retained an intact PB1-F2 gene overexpressed PB1 early in infection and replicated slowly in tissue culture. Thus, the influenza A virus proteome includes a 12th primary translation product that (similarly to PB1-F2) is nonessential for virus viability but whose loss, in particular genetic backgrounds, is detrimental to virus replication.
Figures

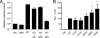
References
-
- Akkina, R. K., J. C. Richardson, M. C. Aguilera, and C. M. Yang. 1991. Heterogeneous forms of polymerase proteins exist in influenza A virus-infected cells. Virus Res. 1917-30. - PubMed
-
- Baigent, S. J., and J. W. McCauley. 2003. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays 25657-671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

