Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive
- PMID: 19494002
- PMCID: PMC2715767
- DOI: 10.1128/JVI.00576-09
Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive
Abstract
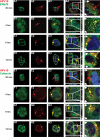
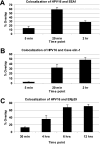
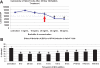
Human papillomavirus type 16 (HPV16) has been identified as being the most common etiological agent leading to cervical cancer. Despite having a clear understanding of the role of HPV16 in oncogenesis, details of how HPV16 traffics during infection are poorly understood. HPV16 has been determined to enter via clathrin-mediated endocytosis, but the subsequent steps of HPV16 infection remain unclear. There is emerging evidence that several viruses take advantage of cross talk between routes of endocytosis. Specifically, JCV and bovine papillomavirus type 1 have been shown to enter cells by clathrin-dependent endocytosis and then require caveolin-1-mediated trafficking for infection. In this paper, we show that HPV16 is dependent on caveolin-1 after clathrin-mediated endocytosis. We provide evidence for the first time that HPV16 infection is dependent on trafficking to the endoplasmic reticulum (ER). This novel trafficking may explain the requirement for the caveolar pathway in HPV16 infection because clathrin-mediated endocytosis typically does not lead to the ER. Our data indicate that the infectious route for HPV16 following clathrin-mediated entry is caveolin-1 and COPI dependent. An understanding of the steps involved in HPV16 sorting and trafficking opens up the possibility of developing novel approaches to interfere with HPV16 infection and reduce the burden of papillomavirus diseases including cervical cancer.
Figures




References
-
- Ahluwalia, N., J. J. Bergeron, I. Wada, E. Degen, and D. B. Williams. 1992. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J. Biol. Chem. 26710914-10918. - PubMed
-
- Bergeron, J. J., M. B. Brenner, D. Y. Thomas, and D. B. Williams. 1994. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 19124-128. - PubMed
-
- Bethune, J., F. Wieland, and J. Moelleken. 2006. COPI-mediated transport. J. Membr. Biol. 21165-79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

