Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus
- PMID: 19494006
- PMCID: PMC2715744
- DOI: 10.1128/JVI.02549-08
Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus
Abstract
The NKG2D receptor is one of the most potent activating natural killer cell receptors involved in antiviral responses. The mouse NKG2D ligands MULT-1, RAE-1, and H60 are regulated by murine cytomegalovirus (MCMV) proteins m145, m152, and m155, respectively. In addition, the m138 protein interferes with the expression of both MULT-1 and H60. We show here that one of five RAE-1 isoforms, RAE-1delta, is resistant to downregulation by MCMV and that this escape has functional importance in vivo. Although m152 retained newly synthesized RAE-1delta and RAE-1gamma in the endoplasmic reticulum, no viral regulator was able to affect the mature RAE-1delta form which remains expressed on the surfaces of infected cells. This differential susceptibility to downregulation by MCMV is not a consequence of faster maturation of RAE-1delta compared to RAE-1gamma but rather an intrinsic property of the mature surface-resident protein. This difference can be attributed to the absence of a PLWY motif from RAE-1delta. Altogether, these findings provide evidence for a novel mechanism of host escape from viral immunoevasion of NKG2D-dependent control.
Figures

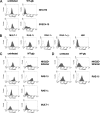
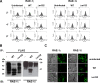

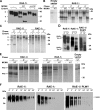
Similar articles
-
Direct interaction of the mouse cytomegalovirus m152/gp40 immunoevasin with RAE-1 isoforms.Biochemistry. 2010 Mar 23;49(11):2443-53. doi: 10.1021/bi902130j. Biochemistry. 2010. PMID: 20166740 Free PMC article.
-
Promiscuity of MCMV immunoevasin of NKG2D: m138/fcr-1 down-modulates RAE-1epsilon in addition to MULT-1 and H60.Mol Immunol. 2009 Nov;47(1):114-22. doi: 10.1016/j.molimm.2009.02.010. Epub 2009 Mar 17. Mol Immunol. 2009. PMID: 19297023
-
Selective down-regulation of the NKG2D ligand H60 by mouse cytomegalovirus m155 glycoprotein.J Virol. 2005 Mar;79(5):2920-30. doi: 10.1128/JVI.79.5.2920-2930.2005. J Virol. 2005. PMID: 15709011 Free PMC article.
-
Murine cytomegalovirus regulation of NKG2D ligands.Med Microbiol Immunol. 2008 Jun;197(2):159-66. doi: 10.1007/s00430-008-0080-7. Epub 2008 Feb 8. Med Microbiol Immunol. 2008. PMID: 18259774 Review.
-
Resisting viral infection: the gene by gene approach.Curr Opin Virol. 2011 Dec;1(6):513-8. doi: 10.1016/j.coviro.2011.10.005. Epub 2011 Nov 6. Curr Opin Virol. 2011. PMID: 22440911 Free PMC article. Review.
Cited by
-
Human cytomegalovirus Fcγ binding proteins gp34 and gp68 antagonize Fcγ receptors I, II and III.PLoS Pathog. 2014 May 15;10(5):e1004131. doi: 10.1371/journal.ppat.1004131. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24830376 Free PMC article.
-
Resilience in Long-Term Viral Infection: Genetic Determinants and Interactions.Int J Mol Sci. 2021 Oct 21;22(21):11379. doi: 10.3390/ijms222111379. Int J Mol Sci. 2021. PMID: 34768809 Free PMC article.
-
How the virus outsmarts the host: function and structure of cytomegalovirus MHC-I-like molecules in the evasion of natural killer cell surveillance.J Biomed Biotechnol. 2011;2011:724607. doi: 10.1155/2011/724607. Epub 2011 Jun 30. J Biomed Biotechnol. 2011. PMID: 21765638 Free PMC article. Review.
-
Modulation of natural killer cell activity by viruses.Curr Opin Microbiol. 2010 Aug;13(4):530-9. doi: 10.1016/j.mib.2010.05.011. Epub 2010 Jun 16. Curr Opin Microbiol. 2010. PMID: 20558100 Free PMC article. Review.
-
NKG2D Receptor and Its Ligands in Host Defense.Cancer Immunol Res. 2015 Jun;3(6):575-82. doi: 10.1158/2326-6066.CIR-15-0098. Cancer Immunol Res. 2015. PMID: 26041808 Free PMC article. Review.
References
-
- Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285727-729. - PubMed
-
- Carayannopoulos, L. N., O. V. Naidenko, D. H. Fremont, and W. M. Yokoyama. 2002. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J. Immunol. 1694079-4083. - PubMed
-
- Carayannopoulos, L. N., O. V. Naidenko, J. Kinder, E. L. Ho, D. H. Fremont, and W. M. Yokoyama. 2002. Ligands for murine NKG2D display heterogeneous binding behavior. Eur. J. Immunol. 32597-605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

