Activation of intracellular signaling pathways by the murine cytomegalovirus G protein-coupled receptor M33 occurs via PLC-{beta}/PKC-dependent and -independent mechanisms
- PMID: 19494016
- PMCID: PMC2715766
- DOI: 10.1128/JVI.02116-08
Activation of intracellular signaling pathways by the murine cytomegalovirus G protein-coupled receptor M33 occurs via PLC-{beta}/PKC-dependent and -independent mechanisms
Abstract
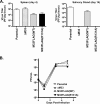
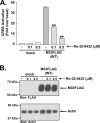
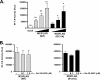
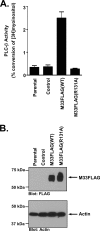
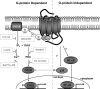
The presence of numerous G protein-coupled receptor (GPCR) homologs within the herpesvirus genomes suggests an essential role for these genes in viral replication in the infected host. Such is the case for murine cytomegalovirus (MCMV), where deletion of the M33 GPCR or replacement of M33 with a signaling defective mutant has been shown to severely attenuate replication in vivo. In the present study we utilized a genetically altered version of M33 (termed R131A) in combination with pharmacological inhibitors to further characterize the mechanisms by which M33 activates downstream signaling pathways. This R131A mutant of M33 fails to support salivary gland replication in vivo and, as such, is an important tool that can be used to examine the signaling activities of M33. We show that M33 stimulates the transcription factor CREB via heterotrimeric G(q/11) proteins and not through promiscuous coupling of M33 to the G(s) pathway. Using inhibitors of signaling molecules downstream of G(q/11), we demonstrate that M33 stimulates CREB transcriptional activity in a phospholipase C-beta and protein kinase C (PKC)-dependent manner. Finally, utilizing wild-type and R131A versions of M33, we show that M33-mediated activation of other signaling nodes, including the mitogen-activated protein kinase family member p38alpha and transcription factor NF-kappaB, occurs in the absence of G(q/11) and PKC signaling. The results from the present study indicate that M33 utilizes multiple mechanisms to modulate intracellular signaling cascades and suggest that signaling through PLC-beta and PKC plays a central role in MCMV pathogenesis in vivo.
Figures

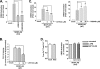
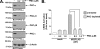
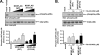
Similar articles
-
The Mouse Cytomegalovirus G Protein-Coupled Receptor Homolog, M33, Coordinates Key Features of In Vivo Infection via Distinct Components of Its Signaling Repertoire.J Virol. 2022 Feb 23;96(4):e0186721. doi: 10.1128/JVI.01867-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878888 Free PMC article.
-
The M33 chemokine receptor homolog of murine cytomegalovirus exhibits a differential tissue-specific role during in vivo replication and latency.J Virol. 2009 Aug;83(15):7590-601. doi: 10.1128/JVI.00386-09. Epub 2009 May 13. J Virol. 2009. PMID: 19439478 Free PMC article.
-
The M33 G protein-coupled receptor encoded by murine cytomegalovirus is dispensable for hematogenous dissemination but is required for growth within the salivary gland.J Virol. 2014 Oct;88(20):11811-24. doi: 10.1128/JVI.01006-14. Epub 2014 Aug 6. J Virol. 2014. PMID: 25100846 Free PMC article.
-
Hijacking GPCRs by viral pathogens and tumor.Biochem Pharmacol. 2016 Aug 15;114:69-81. doi: 10.1016/j.bcp.2016.03.021. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27060663 Free PMC article. Review.
-
Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis.J Clin Virol. 2002 Aug;25 Suppl 2:S111-22. doi: 10.1016/s1386-6532(02)00096-3. J Clin Virol. 2002. PMID: 12361762 Review.
Cited by
-
Constitutive Signaling by the Human Cytomegalovirus G Protein Coupled Receptor Homologs US28 and UL33 Enables Trophoblast Migration In Vitro.Viruses. 2022 Feb 14;14(2):391. doi: 10.3390/v14020391. Viruses. 2022. PMID: 35215985 Free PMC article.
-
Development of a Primary Human Cell Model for the Study of Human Cytomegalovirus Replication and Spread within Salivary Epithelium.J Virol. 2019 Jan 17;93(3):e01608-18. doi: 10.1128/JVI.01608-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404806 Free PMC article.
-
The Mouse Cytomegalovirus G Protein-Coupled Receptor Homolog, M33, Coordinates Key Features of In Vivo Infection via Distinct Components of Its Signaling Repertoire.J Virol. 2022 Feb 23;96(4):e0186721. doi: 10.1128/JVI.01867-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878888 Free PMC article.
-
Lipid metabolism modulation by the P2X7 receptor in the immune system and during the course of infection: new insights into the old view.Purinergic Signal. 2011 Dec;7(4):381-92. doi: 10.1007/s11302-011-9255-6. Epub 2011 Aug 16. Purinergic Signal. 2011. PMID: 21845440 Free PMC article.
-
Methods for Studying the Function of Cytomegalovirus GPCRs.Methods Mol Biol. 2021;2244:159-197. doi: 10.1007/978-1-0716-1111-1_9. Methods Mol Biol. 2021. PMID: 33555587
References
-
- Ahn, S., S. K. Shenoy, H. Wei, and R. J. Lefkowitz. 2004. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 27935518-35525. - PubMed
-
- Alewijnse, A. E., H. Timmerman, E. H. Jacobs, M. J. Smit, E. Roovers, S. Cotecchia, and R. Leurs. 2000. The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol. Pharmacol. 57890-898. - PubMed
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 39186-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

