Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1
- PMID: 19494069
- PMCID: PMC2725685
- DOI: 10.1128/JCM.00232-09
Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1
Abstract
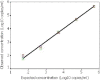
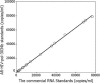
The branched DNA (bDNA) assay is a reliable method for quantifying the RNA of human immunodeficiency virus type 1 (HIV-1). The positive controls and standards for this assay for the detection of HIV-1 consist of naked RNA, which is susceptible to degradation by RNase. Armored RNA is a good candidate for an RNase-resistant positive control or standard. However, its use has been limited by the maximal length of the exogenous RNA packaged into virus-like particles by routine armored RNA technology. In the present study, we produced armored long RNA (armored L-RNA) controls or standards (AR-HIV-pol-3034b) for a bDNA assay of HIV-1 by increasing the amount and affinity of the pac sites (the pac site is a specific 19-nucleotide stem-loop region located at the 5' terminus of the MS2 bacteriophage replicase gene) by a one-plasmid double-expression system. AR-HIV-pol-3034b was completely resistant to DNase and RNase, was stable in normal human EDTA-preserved plasma at 4 degrees C for at least 6 months, and produced reproducible, linear results in the Versant HIV-1 RNA 3.0 assay. In conclusion, AR-HIV-pol-3034b could act as a positive control or standard in a bDNA assay for the detection of HIV-1. In addition, the one-plasmid double-expression system can be used as a better platform than the one-plasmid expression system and the two-plasmid coexpression system for expressing armored L-RNA.
Figures


Similar articles
-
RNase-resistant virus-like particles containing long chimeric RNA sequences produced by two-plasmid coexpression system.J Clin Microbiol. 2008 May;46(5):1734-40. doi: 10.1128/JCM.02248-07. Epub 2008 Feb 27. J Clin Microbiol. 2008. PMID: 18305135 Free PMC article.
-
Construction of armored RNA containing long-size chimeric RNA by increasing the number and affinity of the pac site in exogenous rna and sequence coding coat protein of the MS2 bacteriophage.Intervirology. 2008;51(2):144-50. doi: 10.1159/000141707. Epub 2008 Jul 1. Intervirology. 2008. PMID: 18594159 Free PMC article.
-
Design and Development of a Quantitative TaqMan Real-Time PCR Assay for Evaluation of HIV-1 (group M) Viral Load in Plasma Using Armored RNA Standard.Clin Lab. 2018 Jun 1;64(6):955-963. doi: 10.7754/Clin.Lab.2018.171215. Clin Lab. 2018. PMID: 29945309
-
Molecular-based methods for quantifying HIV viral load.AIDS Patient Care STDS. 2004 Feb;18(2):75-9. doi: 10.1089/108729104322802506. AIDS Patient Care STDS. 2004. PMID: 15006182 Review.
-
The significance of HIV viral load assay precision: a review of the package insert specifications of two commercial kits.J Int Assoc Physicians AIDS Care (Chic). 2002 Oct-Dec;1(4):134-40. doi: 10.1177/154510970200100405. J Int Assoc Physicians AIDS Care (Chic). 2002. PMID: 12942672 Review.
Cited by
-
Optimization of PCR for quantification of simian immunodeficiency virus genomic RNA in plasma of rhesus macaques (Macaca mulatta) using armored RNA.J Med Primatol. 2014 Feb;43(1):31-43. doi: 10.1111/jmp.12088. Epub 2013 Nov 22. J Med Primatol. 2014. PMID: 24266615 Free PMC article.
-
Optimizing the synthesis and purification of MS2 virus like particles.Sci Rep. 2021 Oct 6;11(1):19851. doi: 10.1038/s41598-021-98706-1. Sci Rep. 2021. PMID: 34615923 Free PMC article.
-
Failure to detect Xenotropic murine leukaemia virus-related virus in Chinese patients with chronic fatigue syndrome.Virol J. 2010 Sep 13;7:224. doi: 10.1186/1743-422X-7-224. Virol J. 2010. PMID: 20836869 Free PMC article.
-
Internal control for real-time polymerase chain reaction based on MS2 bacteriophage for RNA viruses diagnostics.Mem Inst Oswaldo Cruz. 2017 May;112(5):339-347. doi: 10.1590/0074-02760160380. Epub 2017 Apr 6. Mem Inst Oswaldo Cruz. 2017. PMID: 28403327 Free PMC article.
-
Simple Low-Cost Production of DNA MS2 Virus-Like Particles As Molecular Diagnostic Controls.GEN Biotechnol. 2022 Dec 1;1(6):496-503. doi: 10.1089/genbio.2022.0033. Epub 2022 Dec 21. GEN Biotechnol. 2022. PMID: 36644571 Free PMC article.
References
-
- Coombs, R. W., S. L. Welles, C. Hooper, P. S. Reichelderfer, R. T. D'Aquila, A. J. Japour, V. A. Johnson, D. R. Kuritzkes, D. D. Richman, S. Kwok, J. Todd, J. B. Jackson, V. DeGruttola, C. S. Crumpacker, J. Kahn, AIDS Clinical Trials Group (ACTG) 116B/117 Study Team, and ACTG Virology Committee Resistance and HIV-1 RNA Working Groups. 1996. Association of plasma human immunodeficiency virus type 1 RNA level with risk of clinical progression in patients with advanced infection. J. Infect. Dis. 174704-712. - PubMed
-
- DuBois, D. B., M. M. Winkler, and B. L. Pasloske. October 1997. Ribonuclease resistant viral RNA standards. U.S. patent 5,677,124.
-
- Greene, W. C. 2007. A history of AIDS: looking back to see ahead. Eur. J. Immunol. 37(Suppl. 1)S94-S102. - PubMed
-
- Horn, W. T., M. A. Convery, N. J. Stonehouse, C. J. Adams, L. Liljas, S. E. Phillips, and P. G. Stockley. 2004. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: further challenges in the modeling of ligand-RNA interactions. RNA 101776-1782. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

