Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1
- PMID: 19494069
- PMCID: PMC2725685
- DOI: 10.1128/JCM.00232-09
Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1
Abstract
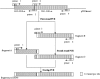
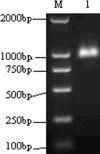
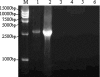
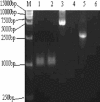
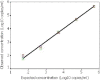
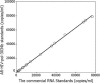
The branched DNA (bDNA) assay is a reliable method for quantifying the RNA of human immunodeficiency virus type 1 (HIV-1). The positive controls and standards for this assay for the detection of HIV-1 consist of naked RNA, which is susceptible to degradation by RNase. Armored RNA is a good candidate for an RNase-resistant positive control or standard. However, its use has been limited by the maximal length of the exogenous RNA packaged into virus-like particles by routine armored RNA technology. In the present study, we produced armored long RNA (armored L-RNA) controls or standards (AR-HIV-pol-3034b) for a bDNA assay of HIV-1 by increasing the amount and affinity of the pac sites (the pac site is a specific 19-nucleotide stem-loop region located at the 5' terminus of the MS2 bacteriophage replicase gene) by a one-plasmid double-expression system. AR-HIV-pol-3034b was completely resistant to DNase and RNase, was stable in normal human EDTA-preserved plasma at 4 degrees C for at least 6 months, and produced reproducible, linear results in the Versant HIV-1 RNA 3.0 assay. In conclusion, AR-HIV-pol-3034b could act as a positive control or standard in a bDNA assay for the detection of HIV-1. In addition, the one-plasmid double-expression system can be used as a better platform than the one-plasmid expression system and the two-plasmid coexpression system for expressing armored L-RNA.
Figures


References
-
- Coombs, R. W., S. L. Welles, C. Hooper, P. S. Reichelderfer, R. T. D'Aquila, A. J. Japour, V. A. Johnson, D. R. Kuritzkes, D. D. Richman, S. Kwok, J. Todd, J. B. Jackson, V. DeGruttola, C. S. Crumpacker, J. Kahn, AIDS Clinical Trials Group (ACTG) 116B/117 Study Team, and ACTG Virology Committee Resistance and HIV-1 RNA Working Groups. 1996. Association of plasma human immunodeficiency virus type 1 RNA level with risk of clinical progression in patients with advanced infection. J. Infect. Dis. 174704-712. - PubMed
-
- DuBois, D. B., M. M. Winkler, and B. L. Pasloske. October 1997. Ribonuclease resistant viral RNA standards. U.S. patent 5,677,124.
-
- Greene, W. C. 2007. A history of AIDS: looking back to see ahead. Eur. J. Immunol. 37(Suppl. 1)S94-S102. - PubMed
-
- Horn, W. T., M. A. Convery, N. J. Stonehouse, C. J. Adams, L. Liljas, S. E. Phillips, and P. G. Stockley. 2004. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: further challenges in the modeling of ligand-RNA interactions. RNA 101776-1782. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

