PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors
- PMID: 19494135
- PMCID: PMC2836830
- DOI: 10.1523/JNEUROSCI.1090-09.2009
PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors
Abstract
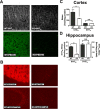
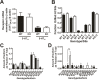
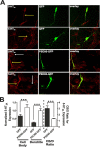
Here, we report that postsynaptic density protein of 95 kDa (PSD-95), a postsynaptic density scaffolding protein, classically conceptualized as being essential for the regulation of ionotropic glutamatergic signaling at the postsynaptic membrane, plays an unanticipated and essential role in mediating the actions of hallucinogens and atypical antipsychotic drugs at 5-HT(2A) and 5-HT(2C) serotonergic G-protein-coupled receptors. We show that PSD-95 is crucial for normal 5-HT(2A) and 5-HT(2C) expression in vivo and that PSD-95 maintains normal receptor expression by promoting apical dendritic targeting and stabilizing receptor turnover in vivo. Significantly, 5-HT(2A)- and 5-HT(2C)-mediated downstream signaling is impaired in PSD-95(null) mice, and the 5-HT(2A)-mediated head-twitch response is abnormal. Furthermore, the ability of 5-HT(2A) inverse agonists to normalize behavioral changes induced by glutamate receptor antagonists is abolished in the absence of PSD-95 in vivo. These results demonstrate that PSD-95, in addition to the well known role it plays in scaffolding macromolecular glutamatergic signaling complexes, profoundly modulates metabotropic 5-HT(2A) and 5-HT(2C) receptor function.
Figures





References
-
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. - PubMed
-
- Ahlemeyer B, Baumgart-Vogt E. Optimized protocols for the simultaneous preparation of primary neuronal cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57BL/6J mice. J Neurosci Methods. 2005;149:110–120. - PubMed
-
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. - PubMed
-
- Bécamel C, Gavarini S, Chanrion B, Alonso G, Galéotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 MH082441/MH/NIMH NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- R01 DA021420/DA/NIDA NIH HHS/United States
- NS057311/NS/NINDS NIH HHS/United States
- R01 NS019576/NS/NINDS NIH HHS/United States
- NS-19576/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R37 MH073853/MH/NIMH NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
- R01 MH073853/MH/NIMH NIH HHS/United States
- MH-73853/MH/NIMH NIH HHS/United States
- R21 NS057311/NS/NINDS NIH HHS/United States
- U19MH82441/MH/NIMH NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- MH61887/MH/NIMH NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- DA021420/DA/NIDA NIH HHS/United States
- R01 MH061887/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
