Scleral biomechanics in the aging monkey eye
- PMID: 19494203
- PMCID: PMC2883469
- DOI: 10.1167/iovs.08-3363
Scleral biomechanics in the aging monkey eye
Abstract
Purpose: To investigate the age-related differences in the inhomogeneous, anisotropic, nonlinear biomechanical properties of posterior sclera from old (22.9 +/- 5.3 years) and young (1.5 +/- 0.7 years) rhesus monkeys.
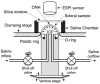
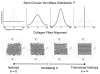
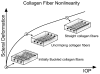
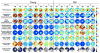
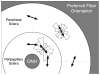
Methods: The posterior scleral shell of each eye was mounted on a custom-built pressurization apparatus, then intraocular pressure (IOP) was elevated from 5 to 45 mm Hg while the 3D displacements of the scleral surface were measured with speckle interferometry. Each scleral shell's geometry was digitally reconstructed from data generated by a 3-D digitizer (topography) and 20-MHz ultrasound (thickness). An inverse finite element (FE) method incorporating a fiber-reinforced constitutive model was used to extract a unique set of biomechanical properties for each eye. Displacements, thickness, stress, strain, tangent modulus, structural stiffness, and preferred collagen fiber orientation were mapped for each posterior sclera.
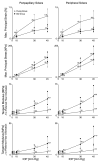
Results: The model yielded 3-D deformations of posterior sclera that matched well with those observed experimentally. The posterior sclera exhibited inhomogeneous, anisotropic, nonlinear mechanical behavior. The sclera was significantly thinner (P = 0.038) and tangent modulus and structural stiffness were significantly higher in old monkeys (P < 0.0001). On average, scleral collagen fibers were circumferentially oriented around the optic nerve head (ONH). No difference was found in the preferred collagen fiber orientation and fiber concentration factor between age groups.
Conclusions: Posterior sclera of old monkeys is significantly stiffer than that of young monkeys and is therefore subject to higher stresses but lower strains at all levels of IOP. Age-related stiffening of the sclera may significantly influence ONH biomechanics and potentially contribute to age-related susceptibility to glaucomatous vision loss.
Figures

References
-
- Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005;19:1133–1135. - PubMed
-
- Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15:606–616. - PubMed
-
- Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. - PubMed
-
- Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000;41:2991–3000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

