IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge
- PMID: 19494257
- PMCID: PMC2724021
- DOI: 10.4049/jimmunol.0900657
IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge
Abstract
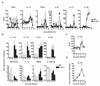
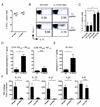
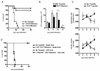
We examined the expression and influence of IL-10 during influenza infection. We found that IL-10 does not impact sublethal infection, heterosubtypic immunity, or the maintenance of long-lived influenza Ag depots. However, IL-10-deficient mice display dramatically increased survival compared with wild-type mice when challenged with lethal doses of virus, correlating with increased expression of several Th17-associated cytokines in the lungs of IL-10-deficient mice during the peak of infection, but not with unchecked inflammation or with increased cellular responses. Foxp3(-) CD4 T cell effectors at the site of infection represent the most abundant source of IL-10 in wild-type mice during high-dose influenza infection, and the majority of these cells coproduce IFN-gamma. Finally, compared with predominant Th1 responses in wild-type mice, virus-specific T cell responses in the absence of IL-10 display a strong Th17 component in addition to a strong Th1 response and we show that Th17-polarized CD4 T cell effectors can protect naive mice against an otherwise lethal influenza challenge and utilize unique mechanisms to do so. Our results show that IL-10 expression inhibits development of Th17 responses during influenza infection and that this is correlated with compromised protection during high-dose primary, but not secondary, challenge.
Figures




References
-
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
-
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. - PubMed
-
- Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

