Modulation of p38 MAPK activity in regulatory T cells after tolerance with anti-DNA Ig peptide in (NZB x NZW)F1 lupus mice
- PMID: 19494264
- PMCID: PMC2758643
- DOI: 10.4049/jimmunol.0804214
Modulation of p38 MAPK activity in regulatory T cells after tolerance with anti-DNA Ig peptide in (NZB x NZW)F1 lupus mice
Abstract
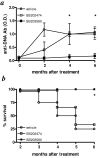
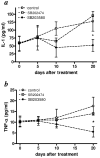
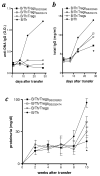
Treatment of (NZB x NZW)F(1) (NZB/W) lupus-prone mice with the anti-DNA Ig-based peptide pConsensus prolongs the survival of treated animals and effectively delays the appearance of autoantibodies and glomerulonephritis. We have previously shown that part of these protective effects associated with the induction of CD4(+)CD25(+)Foxp3(+) regulatory T cells (Tregs) that suppressed autoantibody responses. Because the effects of pConsensus appeared secondary to qualitative rather than quantitative changes in Tregs, we investigated the molecular events induced by tolerance in Tregs and found that signaling pathways including ZAP70, p27, STAT1, STAT3, STAT6, SAPK, ERK, and JNK were not significantly affected. However, peptide tolerization affected in Tregs the activity of the MAPK p38, whose phosphorylation was reduced by tolerance. The pharmacologic inhibition of p38 with the pyridinyl imidazole inhibitor SB203580 in naive NZB/W mice reproduced in vivo the effects of peptide-induced tolerance and protected mice from lupus-like disease. Transfer experiments confirmed the role of p38 in Tregs on disease activity in the NZB/W mice. These data indicate that the modulation of p38 activity in lupus Tregs can significantly influence the disease activity.
Figures



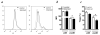

References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. - PubMed
-
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. - PubMed
-
- Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. - PubMed
-
- La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

