The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity
- PMID: 19494265
- PMCID: PMC2694955
- DOI: 10.4049/jimmunol.0900075
The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity
Abstract
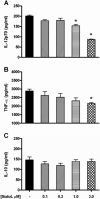
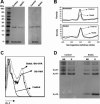
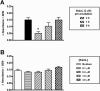
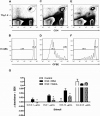
Sialostatin L (SialoL) is a secreted cysteine protease inhibitor identified in the salivary glands of the Lyme disease vector Ixodes scapularis. In this study, we reveal the mechanisms of SialoL immunomodulatory actions on the vertebrate host. LPS-induced maturation of dendritic cells from C57BL/6 mice was significantly reduced in the presence of SialoL. Although OVA degradation was not affected by the presence of SialoL in dendritic cell cultures, cathepsin S activity was partially inhibited, leading to an accumulation of a 10-kDa invariant chain intermediate in these cells. As a consequence, in vitro Ag-specific CD4(+) T cell proliferation was inhibited in a time-dependent manner by SialoL, and further studies engaging cathepsin S(-/-) or cathepsin L(-/-) dendritic cells confirmed that the immunomodulatory actions of SialoL are mediated by inhibition of cathepsin S. Moreover, mice treated with SialoL displayed decreased early T cell expansion and recall response upon antigenic stimulation. Finally, SialoL administration during the immunization phase of experimental autoimmune encephalomyelitis in mice significantly prevented disease symptoms, which was associated with impaired IFN-gamma and IL-17 production and specific T cell proliferation. These results illuminate the dual mechanism by which a human disease vector protein modulates vertebrate host immunity and reveals its potential in prevention of an autoimmune disease.
Figures


References
-
- Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990;6:157–160. - PubMed
-
- Hill CA, Wikel SK. The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. - PubMed
-
- Center for Disease Control and Prevention. Lyme disease - United States, 2001−2002. Morb Mortal Wkly Rep. 2004;53:107–113.
-
- Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J Med Entomol. 1992;29:818–826. - PubMed
-
- Zeidner N, Mbow ML, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

