Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway
- PMID: 19494325
- PMCID: PMC2787095
- DOI: 10.4049/jimmunol.0802884
Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway
Abstract
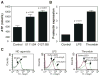
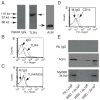
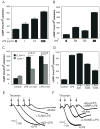
Bacterial LPS induces rapid thrombocytopenia, hypotension, and sepsis. Although growing evidence suggests that platelet activation plays a critical role in LPS-induced thrombocytopenia and tissue damage, the mechanism of LPS-mediated platelet activation is unclear. In this study, we show that LPS stimulates platelet secretion of dense and alpha granules as indicated by ATP release and P-selectin expression, and thus enhances platelet activation induced by low concentrations of platelet agonists. Platelets express components of the LPS receptor-signaling complex, including TLR (TLR4), CD14, MD2, and MyD88, and the effect of LPS on platelet activation was abolished by an anti-TLR4-blocking Ab or TLR4 knockout, suggesting that the effect of LPS on platelet aggregation requires the TLR4 pathway. Furthermore, LPS-potentiated thrombin- and collagen-induced platelet aggregation and FeCl(3)-induced thrombus formation were abolished in MyD88 knockout mice. LPS also induced cGMP elevation and the stimulatory effect of LPS on platelet aggregation was abolished by inhibitors of NO synthase and the cGMP-dependent protein kinase (PKG). LPS-induced cGMP elevation was inhibited by an anti-TLR4 Ab or by TLR4 deficiency, suggesting that activation of the cGMP/protein kinase G pathway by LPS involves the TLR4 pathway. Taken together, our data indicate that LPS stimulates platelet secretion and potentiates platelet aggregation through a TLR4/MyD88- and cGMP/PKG-dependent pathway.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



References
-
- Tobias PS, Mathison JC, Ulevitch RJ. A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J Biol Chem. 1988;263:13479–13481. - PubMed
-
- Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. - PubMed
-
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

