Effects of corticosteroids on osteopontin expression in a murine model of allergic asthma
- PMID: 19494498
- PMCID: PMC2844795
- DOI: 10.1159/000210647
Effects of corticosteroids on osteopontin expression in a murine model of allergic asthma
Abstract
Background: Osteopontin (OPN) contributes to the development of T helper 1 (Th1)-mediated immunity and Th1-associated diseases. However, the role of OPN in bronchial asthma is unclear. Corticosteroids reduce airway inflammation, as reflected by the low eosinophil and T-cell counts, and the low level of cytokine expression. We investigated OPN production and the inhibitory effects of corticosteroids on OPN production in a murine model of allergic asthma.
Methods: BALB/c mice were sensitized by intraperitoneal injections of ovalbumin (OVA) with alum. Some mice received daily injections of dexamethasone (DEX) or phosphate-buffered saline for 1 week. All OVA-challenged mice were exposed to aerosolized 1% OVA for 30 min an hour after these injections. After the OVA challenge, the mice were killed, and bronchoalveolar lavage (BAL) fluid and lung tissue were examined.
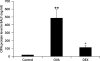
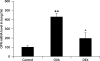
Results: The levels of OPN protein in BAL fluid and OPN mRNA in lung tissue increased after OVA challenge. Most OPN-expressing cells were CD11c+ cells and some were T cells. DEX decreased the levels of OPN protein in the BAL fluid, and those of OPN mRNA and OPN protein in lung tissue.
Conclusions: OPN may play an important role in allergic bronchial asthma. Corticosteroids inhibit OPN production in mice with allergic asthma. The beneficial effect of corticosteroids in bronchial asthma is partly due to their inhibitory effects on OPN production.
Copyright 2009 S. Karger AG, Basel.
Figures




Similar articles
-
Effects of corticosteroid on the expression of thymus and activation-regulated chemokine in a murine model of allergic asthma.Int Arch Allergy Immunol. 2005;137 Suppl 1:60-8. doi: 10.1159/000085434. Epub 2005 Jun 2. Int Arch Allergy Immunol. 2005. PMID: 15947487
-
[Effect of dexamethasone on osteopontin expression in the lung tissue of asthmatic mice].Zhongguo Dang Dai Er Ke Za Zhi. 2014 Dec;16(12):1265-70. Zhongguo Dang Dai Er Ke Za Zhi. 2014. PMID: 25523578 Chinese.
-
Enhanced osteopontin expression in a murine model of allergen-induced airway remodelling.Clin Exp Allergy. 2007 Oct;37(10):1444-54. doi: 10.1111/j.1365-2222.2007.02801.x. Clin Exp Allergy. 2007. PMID: 17883724
-
Galectin-9 in allergic airway inflammation and hyper-responsiveness in mice.Int Arch Allergy Immunol. 2010;151(4):308-17. doi: 10.1159/000250439. Epub 2009 Oct 22. Int Arch Allergy Immunol. 2010. PMID: 19851072
-
Expression of interleukin-17F in a mouse model of allergic asthma.Int Arch Allergy Immunol. 2007;143 Suppl 1:89-94. doi: 10.1159/000101413. Epub 2007 May 1. Int Arch Allergy Immunol. 2007. PMID: 17541285
Cited by
-
Osteopontin contributes to late-onset asthma phenotypes in adult asthma patients.Exp Mol Med. 2020 Feb;52(2):253-265. doi: 10.1038/s12276-020-0376-2. Epub 2020 Feb 3. Exp Mol Med. 2020. PMID: 32009132 Free PMC article.
-
Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus.Arthritis Res Ther. 2013 Jan 23;15(1):R18. doi: 10.1186/ar4150. Arthritis Res Ther. 2013. PMID: 23343383 Free PMC article.
-
Quantitative expression of osteopontin in nasal mucosa of patients with allergic rhinitis: effects of pollen exposure and nasal glucocorticoid treatment.Allergy Asthma Clin Immunol. 2010 Nov 2;6(1):28. doi: 10.1186/1710-1492-6-28. Allergy Asthma Clin Immunol. 2010. PMID: 21044308 Free PMC article.
-
Expression profile of the matricellular protein osteopontin in primary open-angle glaucoma and the normal human eye.Invest Ophthalmol Vis Sci. 2011 Aug 16;52(9):6443-51. doi: 10.1167/iovs.11-7409. Invest Ophthalmol Vis Sci. 2011. PMID: 21743018 Free PMC article.
-
Circulating Clusterin and Osteopontin Levels in Asthma and Asthmatic Pregnancy.Can Respir J. 2017;2017:1602039. doi: 10.1155/2017/1602039. Epub 2017 Oct 23. Can Respir J. 2017. PMID: 29200898 Free PMC article.
References
-
- O'Regan A. The role of osteopontin in the lung disease. Cytokine Growth Factor Rev. 2003;14:479–488. - PubMed
-
- Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhart DT, McCulloch CA, Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167. - PubMed
-
- Higuchi Y, Tamura Y, Uchida T, Matsuura K, Hijiya N, Yamamoto S. The roles of soluble osteopontin using osteopontin-transgenic mice in vivo: proliferation of CD4+ T lymphocytes and the enhancement of cell-mediated immune responses. Pathobiology. 2004;71:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

