Autoinducer-2-based chemical communication in bacteria: complexities of interspecies signaling
- PMID: 19494577
- PMCID: PMC3042238
- DOI: 10.1159/000219371
Autoinducer-2-based chemical communication in bacteria: complexities of interspecies signaling
Abstract
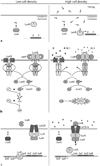
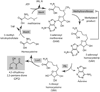
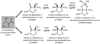
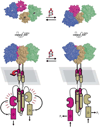
Cell-cell communication in bacteria, called quorum sensing, relies on production, release, and detection of signaling molecules, termed autoinducers. Communication enables populations of cells to synchronize gene expression and therefore behave as a group in a manner akin to cells in multicellular organisms. Most quorum-sensing systems allow communication within an individual species of bacteria. However, one autoinducer, called AI-2, is produced and recognized by many different bacterial species, indicating that some bacteria communicate across species boundaries. Current studies are aimed at discovering the role that AI-2 plays in gene regulation. Differential gene expression in response to AI-2 may cause bacterial behavioral changes, such as biofilm formation or transition to a pathogenic state. Interestingly, multiple mechanisms to detect AI-2 exist. These differences likely reflect variations in the role that AI-2 plays for different bacteria. Additionally, structural analyses of the AI-2 receptor in V. harveyi have provided insight into bacterial trans-membrane signal transduction. A further understanding of bacterial quorum-sensing processes may facilitate development of new technologies aimed at interfering with bacterial communication and virulence.
Copyright (c) 2009 S. Karger AG, Basel.
Figures
Similar articles
-
LuxS quorum sensing: more than just a numbers game.Curr Opin Microbiol. 2003 Apr;6(2):191-7. doi: 10.1016/s1369-5274(03)00028-6. Curr Opin Microbiol. 2003. PMID: 12732311 Review.
-
Saccharomyces cerevisiae Requires CFF1 To Produce 4-Hydroxy-5-Methylfuran-3(2H)-One, a Mimic of the Bacterial Quorum-Sensing Autoinducer AI-2.mBio. 2021 Mar 9;12(2):e03303-20. doi: 10.1128/mBio.03303-20. mBio. 2021. PMID: 33688008 Free PMC article.
-
Recent progresses on AI-2 bacterial quorum sensing inhibitors.Curr Med Chem. 2012;19(2):174-86. doi: 10.2174/092986712803414187. Curr Med Chem. 2012. PMID: 22320296 Review.
-
The intragenus and interspecies quorum-sensing autoinducers exert distinct control over Vibrio cholerae biofilm formation and dispersal.PLoS Biol. 2019 Nov 11;17(11):e3000429. doi: 10.1371/journal.pbio.3000429. eCollection 2019 Nov. PLoS Biol. 2019. PMID: 31710602 Free PMC article.
-
The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule.Mol Microbiol. 2001 Jul;41(2):463-76. doi: 10.1046/j.1365-2958.2001.02532.x. Mol Microbiol. 2001. PMID: 11489131
Cited by
-
Regulation of Toxin Production in Clostridium perfringens.Toxins (Basel). 2016 Jul 5;8(7):207. doi: 10.3390/toxins8070207. Toxins (Basel). 2016. PMID: 27399773 Free PMC article. Review.
-
Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism.Mol Microbiol. 2011 Jan;79(1):7-20. doi: 10.1111/j.1365-2958.2010.07455.x. Epub 2010 Nov 18. Mol Microbiol. 2011. PMID: 21166890 Free PMC article. Review.
-
Stress in the microbiome-immune crosstalk.Gut Microbes. 2024 Jan-Dec;16(1):2327409. doi: 10.1080/19490976.2024.2327409. Epub 2024 Mar 15. Gut Microbes. 2024. PMID: 38488630 Free PMC article. Review.
-
The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems.Front Microbiol. 2017 Jan 20;8:37. doi: 10.3389/fmicb.2017.00037. eCollection 2017. Front Microbiol. 2017. PMID: 28163702 Free PMC article. Review.
-
The impact of quorum sensing on the modulation of phage-host interactions.J Bacteriol. 2021 May 1;203(9):e00687-20. doi: 10.1128/JB.00687-20. Epub 2021 Jan 19. J Bacteriol. 2021. PMID: 33468597 Free PMC article. Review.
References
-
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. - PubMed
-
- Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. - PubMed
-
- Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. - PubMed
-
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

