Heme oxygenase-1 accelerates cutaneous wound healing in mice
- PMID: 19495412
- PMCID: PMC2686151
- DOI: 10.1371/journal.pone.0005803
Heme oxygenase-1 accelerates cutaneous wound healing in mice
Abstract
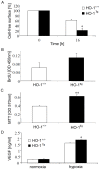
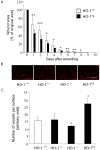
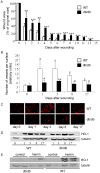
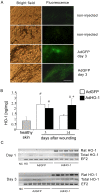
Heme oxygenase-1 (HO-1), a cytoprotective, pro-angiogenic and anti-inflammatory enzyme, is strongly induced in injured tissues. Our aim was to clarify its role in cutaneous wound healing. In wild type mice, maximal expression of HO-1 in the skin was observed on the 2(nd) and 3(rd) days after wounding. Inhibition of HO-1 by tin protoporphyrin-IX resulted in retardation of wound closure. Healing was also delayed in HO-1 deficient mice, where lack of HO-1 could lead to complete suppression of reepithelialization and to formation of extensive skin lesions, accompanied by impaired neovascularization. Experiments performed in transgenic mice bearing HO-1 under control of keratin 14 promoter showed that increased level of HO-1 in keratinocytes is enough to improve the neovascularization and hasten the closure of wounds. Importantly, induction of HO-1 in wounded skin was relatively weak and delayed in diabetic (db/db) mice, in which also angiogenesis and wound closure were impaired. In such animals local delivery of HO-1 transgene using adenoviral vectors accelerated the wound healing and increased the vascularization. In summary, induction of HO-1 is necessary for efficient wound closure and neovascularization. Impaired wound healing in diabetic mice may be associated with delayed HO-1 upregulation and can be improved by HO-1 gene transfer.
Conflict of interest statement
Figures
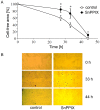
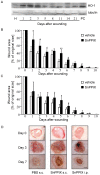
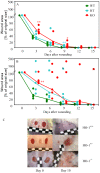
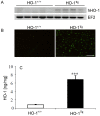
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. - PubMed
-
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S–38S. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. - PubMed
-
- Michaels J, Dobryansky M, Galiano RD, Bhatt KA, Ashinoff R, et al. Topical vascular endothelial growth factor reverses delayed wound healing secondary to angiogenesis inhibitor administration. Wound Repair Regen. 2005;13:506–512. - PubMed
-
- Bloch W, Huggel K, Sasaki T, Grose R, Bugnon P, et al. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. Faseb J. 2000;14:2373–2376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

