Mural cell associated VEGF is required for organotypic vessel formation
- PMID: 19495422
- PMCID: PMC2688382
- DOI: 10.1371/journal.pone.0005798
Mural cell associated VEGF is required for organotypic vessel formation
Abstract
Background: Blood vessels comprise endothelial cells, mural cells (pericytes/vascular smooth muscle cells) and basement membrane. During angiogenesis, mural cells are recruited to sprouting endothelial cells and define a stabilizing context, comprising cell-cell contacts, secreted growth factors and extracellular matrix components, that drives vessel maturation and resistance to anti-angiogenic therapeutics.
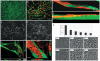
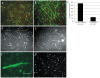
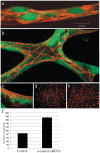
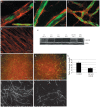
Methods and findings: To better understand the basis for mural cell regulation of angiogenesis, we conducted high content imaging analysis on a microtiter plate format in vitro organotypic blood vessel system comprising primary human endothelial cells co-cultured with primary human mural cells. We show that endothelial cells co-cultured with mural cells undergo an extensive series of phenotypic changes reflective of several facets of blood vessel formation and maturation: Loss of cell proliferation, pathfinding-like cell migration, branching morphogenesis, basement membrane extracellular matrix protein deposition, lumen formation, anastamosis and development of a stabilized capillary-like network. This phenotypic sequence required endothelial-mural cell-cell contact, mural cell-derived VEGF and endothelial VEGFR2 signaling. Inhibiting formation of adherens junctions or basement membrane structures abrogated network formation. Notably, inhibition of mural cell VEGF expression could not be rescued by exogenous VEGF.
Conclusions: These results suggest a unique role for mural cell-associated VEGF in driving vessel formation and maturation.
Conflict of interest statement
Figures

Similar articles
-
Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness.FASEB J. 2001 Feb;15(2):447-57. doi: 10.1096/fj.00-0139com. FASEB J. 2001. PMID: 11156960
-
CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis.PLoS One. 2012;7(2):e30562. doi: 10.1371/journal.pone.0030562. Epub 2012 Feb 20. PLoS One. 2012. PMID: 22363445 Free PMC article.
-
Screening assay for blood vessel maturation inhibitors.Biochem Biophys Res Commun. 2013 Aug 23;438(2):364-9. doi: 10.1016/j.bbrc.2013.07.077. Epub 2013 Jul 25. Biochem Biophys Res Commun. 2013. PMID: 23892038 Free PMC article.
-
Mechanisms of ocular angiogenesis and its molecular mediators.Dev Ophthalmol. 2010;46:4-20. doi: 10.1159/000320006. Epub 2010 Aug 10. Dev Ophthalmol. 2010. PMID: 20703029 Review.
-
Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis.Circ Res. 2008 Mar 28;102(6):637-52. doi: 10.1161/CIRCRESAHA.107.167171. Circ Res. 2008. PMID: 18369162 Review.
Cited by
-
Mesenchymal stem cells induce endothelial cell quiescence and promote capillary formation.Stem Cell Res Ther. 2014 Feb 17;5(1):23. doi: 10.1186/scrt412. Stem Cell Res Ther. 2014. PMID: 24533904 Free PMC article.
-
Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments.Biomaterials. 2020 Jun;243:119921. doi: 10.1016/j.biomaterials.2020.119921. Epub 2020 Mar 6. Biomaterials. 2020. PMID: 32172030 Free PMC article.
-
Manipulating the microvasculature and its microenvironment.Crit Rev Biomed Eng. 2013;41(2):91-123. doi: 10.1615/critrevbiomedeng.2013008077. Crit Rev Biomed Eng. 2013. PMID: 24580565 Free PMC article. Review.
-
Vascular endothelial growth factor gene polymorphisms and the risk of renal cell carcinoma: Evidence from eight case-control studies.Oncotarget. 2017 Jan 31;8(5):8447-8458. doi: 10.18632/oncotarget.14263. Oncotarget. 2017. PMID: 28039484 Free PMC article. Review.
-
Digital Light Processing 3D Bioprinting of Gelatin-Norbornene Hydrogel for Enhanced Vascularization.Macromol Biosci. 2023 Dec;23(12):e2300213. doi: 10.1002/mabi.202300213. Epub 2023 Aug 9. Macromol Biosci. 2023. PMID: 37536347 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

